CytoNiche: New Mode of large-scale Cell Culture CDMO Based on 3D Microcarrier Technology
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-23
- Views:590
(Summary description)In August 2022, CytoNiche 3D Cell Intelligent Manufacturing and Regeneration Medical Center—3D FloTrix®Cell CDMO Platform was officially unveiled, adding a new CDMO service platform in the field of ce
CytoNiche: New Mode of large-scale Cell Culture CDMO Based on 3D Microcarrier Technology
(Summary description)In August 2022, CytoNiche 3D Cell Intelligent Manufacturing and Regeneration Medical Center—3D FloTrix®Cell CDMO Platform was officially unveiled, adding a new CDMO service platform in the field of ce
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-23
- Views:590
In August 2022, CytoNiche 3D Cell Intelligent Manufacturing and Regeneration Medical Center—3D FloTrix®Cell CDMO Platform was officially unveiled, adding a new CDMO service platform in the field of cell therapy. CytoNiche CEO sent a clear message: "We are not here for 'involution'...". Then he explained unhurriedly why he said so.

Dr. Liu Wei, co-founder & CEO of CytoNiche
【Origin】
The origin of CytoNiche can be traced back to 2010. That year, Mr. Du Yanan who completed his post-doctoral work at Harvard Medical School and MIT and returned to China to teach met Liu Wei and Yan Xiaojun who had just entered their doctoral program. When setting up their project, they thought of the stem cell that was in the new wave of R&D at that time, and proposed "making something" to provide the stem cell a "nest" and a "home". At the beginning, they made an in vivo application, that is, they made a cell delivery system which could accurately deliver the cells to the treatment site.
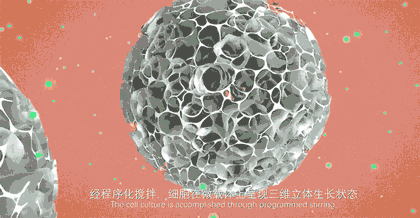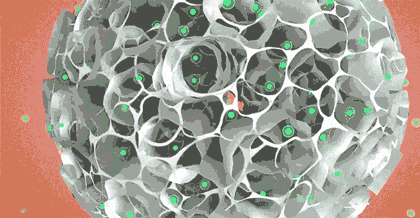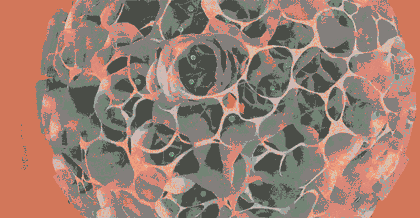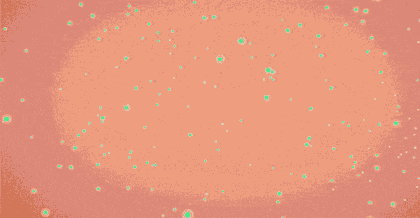
"It was just this idea at that time." Liu Wei, CEO of CytoNiche, recalled. The original intention of the research was only to culture stem cells well in vitro and deliver them accurately in vivo. In order to achieve the goal, under the leadership of Professor Du, they completed this cell "nest"——3D microcarrier by using interdisciplinary technical means such as biomaterials and regeneration medicine, and realized culture in vitro and delivery in vivo. From the end of 2014 to the beginning of 2015, they published a series of internationally renowned papers, which were highly recognized by academic circles, leading to the proposal of the concept "stem cell pharmacy" for the first time.
"At that time, it was recognized by many that cells would be marketed as 'medicine' in the future." Although it was not defined as "medicine" in China at that time, it had already been developed internationally in accordance with the mode and standards of "medicine". Well, as "medicine", standard production and manufacturing, adjuvants, delivery systems, etc. are required for cells.
They combined 3D microcarrier with cell "medicine" in the research to form 3D microtissue, and found that after using 3D microtissue, only one tenth of the cell dose was sufficient to achieve the therapeutic effect of the original number of pure free cells, and the targeted aggregation and targeting effect were obvious.
With these accumulations, they began to think about how to get out of the laboratory and carry out transformation and application.
However, at that time, there were no laws and regulations related to stem cells in China. It was extremely difficult for a complex product with cells combined with biomaterials to take the path of drug declaration, which required a long time and long-term work——"even more than 10 years." Liu Wei said frankly, "It's very difficult to raise funds if your commercialization takes such a long time!"
Then, what can be done in the short term?
【Efficient and high-quality cell culture】
Considering that 3D microcarrier can keep cells active in vivo and exert repair and regeneration effects for a long time, will they also improve the culture efficiency of cells in vitro? So they adjusted the direction and began the research into transformation and application by taking it as a cell culture medium and a consumable for culture.
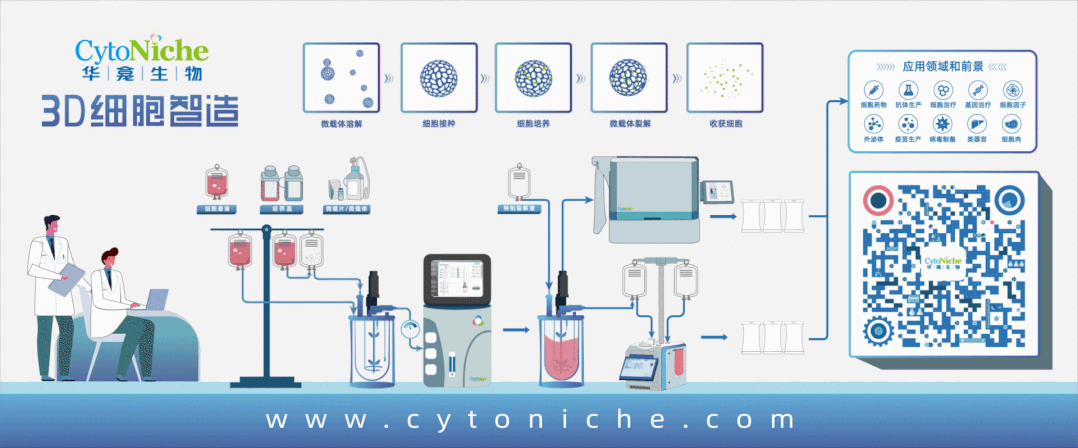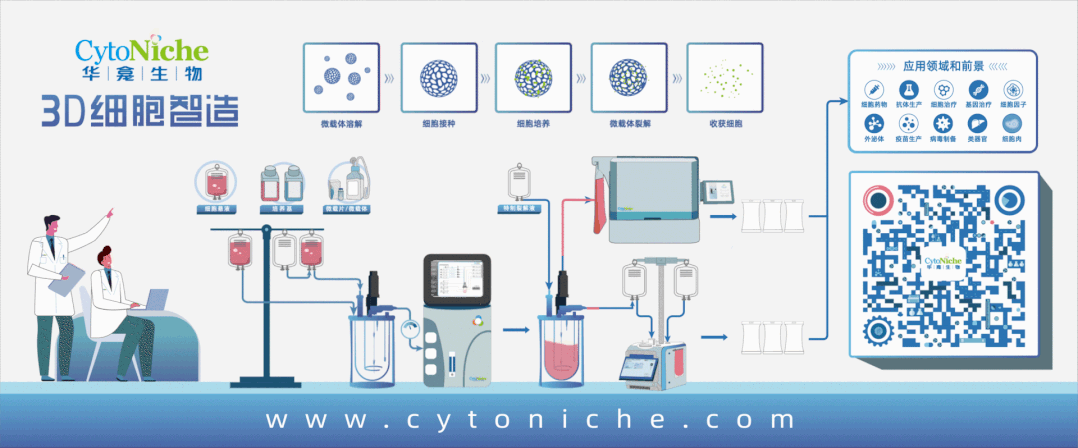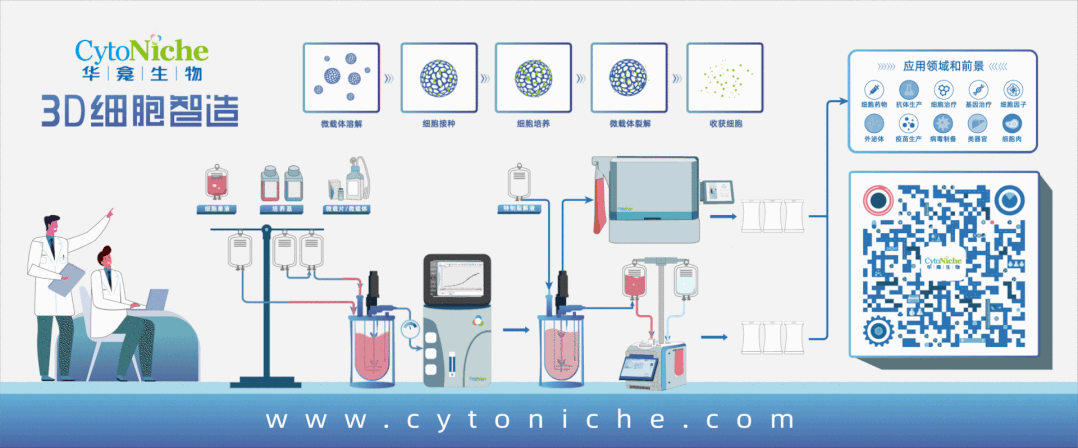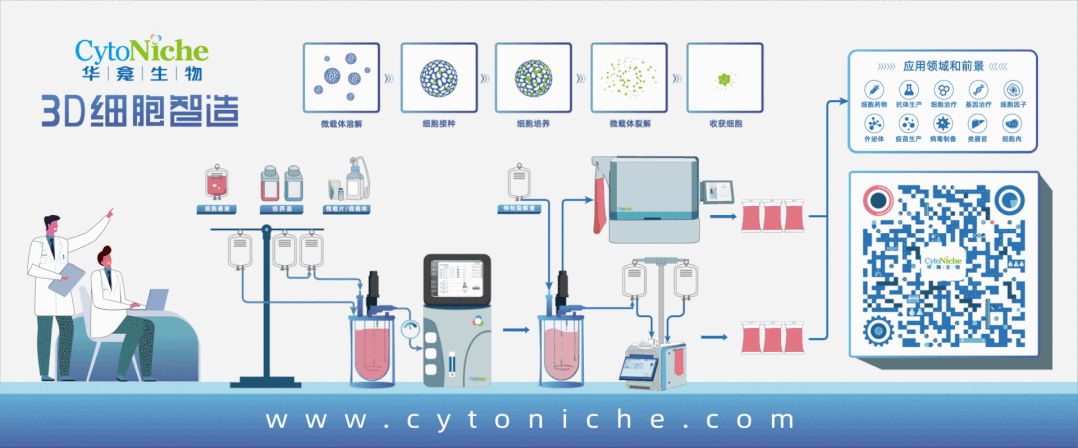
Taking stem cell as an example, traditional stem cell culture relies on artificial "planting" of stem cells on culture dishes or culture flasks. One T75 culture flask can culture 4-6 million stem cells at most, and several T75 culture flasks of stem cells are required for one therapy. Artificial culture and traditional harvesting methods lead to problems such as high cost of cell preparation, low yield of single batch, unstable quality across batches, and no standardization of process flow, which limit the industrial application. However, 3D microcarrier is three-dimensional, and it can swell into tens of thousands of spherical particles when exposed to water. Innumerable pores are densely distributed on the surface of the particles, just like a honeycomb. Stem cells can adhere to each pore for growth and amplification. These honeycomb-shaped particles significantly increase the specific surface area of the carriers where stem cells grow, and the number of stem cells cultured is exponentially increased compared with that in the traditional way.

If the traditional two-dimensional plane culture is regarded as a "manual workshop", 3D microcarrier is a "modern factory". 3D microcarrier can not only significantly improve the cell amplification efficiency, but also adjust the pore diameter and hardness of the carrier according to the characteristics of different cells, thus realizing customized culture for different kinds of cells. It provides a culture environment close to the body for cells, and realize bionic and high-quality cell proliferation.
After the cells are cultured, here comes the harvest. The traditional harvesting method of trypsin digestion is very easy to cause damage to the cell quality, and difficult to achieve harvesting without damage——unless the cultured cells are particularly "sturdy" and tough. Therefore, this method is more applied in the fields taking the non-cell as the end product, such as cell production involved in vaccines and antibodies.
The targeted end product of 3D microcarrier development is the cell, so it is very important to harvest cells safely and without damage. Another characteristic of 3D microcarrier plays an important role——degradability. When harvesting cells, the supporting materials can be degraded and the cells can be released with the help of customized "mild" lysis solution, so as to achieve efficient and mild harvesting of all cells and avoid the damage to cells caused by traditional trypsin digestion solution. Through routine and simple cleaning, it can be ensured that no harmful substances remain in the end product, which meets the quality standards and safety requirements of the end product of cell preparation.
【With engineering mentality, make equipment with soul by themselves】
At this point, the development of an efficient and high-quality cell culture consumable seems to have been completed.
However, if 3D microcarrier is the carrier of cell culture, what is the carrier of the 3D microcarrier?
In the laboratory, many operations can be performed manually, but equipment is required for industrialized large-scale cell culture.
"At first, we also wanted to cooperate with equipment suppliers." They began to look for suitable equipment suppliers, but after a round of searching, they found that due to the innovativeness of 3D microcarrier, there were some special processing requirements in the cell culture process that the existing equipment cannot meet. Both hardware and software of the equipment required a lot of changes.
Therefore, this engineering team from Tsinghua University, adhering to the engineering mentality, determined to build its own equipment R&D team.
"Equipment requires a soul." Liu Wei stressed that "the soul is the process!" Equipment is only the means to realize the process flow and the end product. Only the equipment developed and designed for the special process has a real soul.
It is natural for them who are well versed in these special processes and have engineering background to develop a set of automation equipment to meet the production needs of large-scale cell culture.
"It took us about three years to successfully increase the microcarrier yield from milligram level to kilogram level around the end of 2017." Behind this account of several words are countless explorations and efforts. Du Yanan, who was an undergraduate at the Department of Chemical Engineering in Tsinghua University, led the team to make more use of the principles of chemical engineering, amplify and control the process step by step, and gradually build a production system starting from each screw and each pipeline, which eventually met the requirements for mass production.
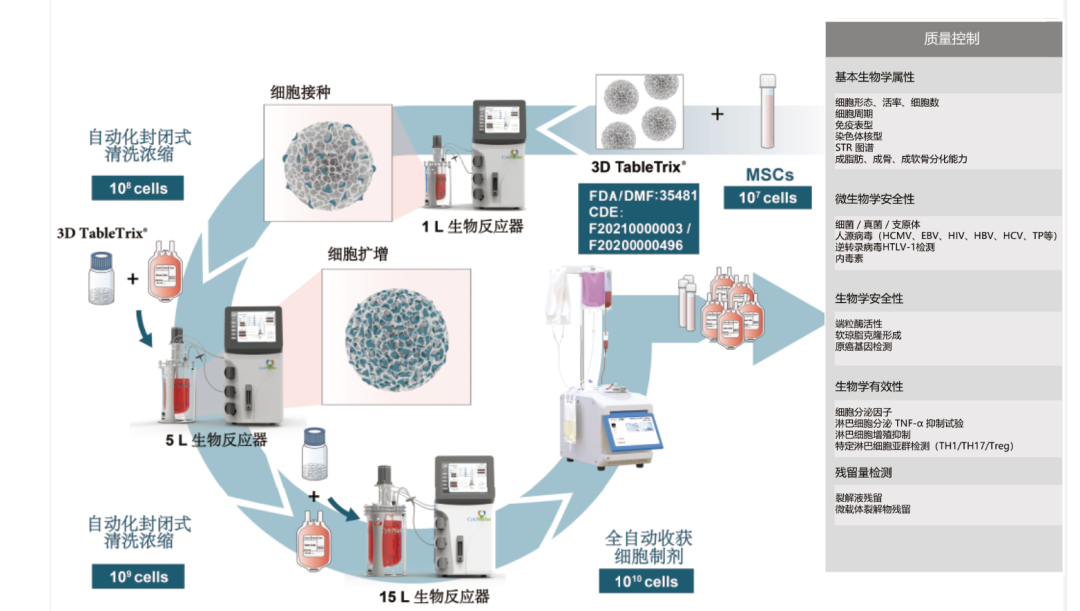
The bioreactor adapted to 3D microcarrier does not require complicated and cumbersome manual operations. It only requires to put 3D microcarrier and cell suspension into the reactor to achieve efficient large-scale amplification and culture of cells. Combined with the cell harvesting system, it can realize fully-enclosed cell harvesting, cleaning, concentration and packaging.
At this point, a whole closed-loop process centering around the cell culture consumable 3D microcarrier has been formed.
【Step out of the lab, step into the cell "intelligent manufacturing"】
In August, 2018, with the assistance of Office of Technology Transfer, Tsinghua University, Du Yanan and team members founded Beijing CytoNiche Biotechnology Co., Ltd. At that point, scientific research achievements officially left the laboratory and stepped into the industry.


At the end of 2017, CFDA released Technical Guidelines for Research and Evaluation of Cell Therapy Products (Trial). In China, cells began to develop into "medicine", and the research and development of cell medicine have been increasing ever since. It is foreseeable that in the future, there will be continuous research and development, marketing and clinical application of cell medicine in China.
"When it comes to medicine development, people may pay more attention to clinical effectiveness and druggability, and then think about mass production." According to Liu Wei, this is probably the normal thinking of most people. However, this "normal thinking" led to the foreign monopoly of the high value-added consumables and equipment in the upstream in the process of many innovative medicine research and development in China, and finally in the mass production.
So, breaking this monopoly in the field of cell medicine is the "original intention" of CytoNiche.

Just like the meaning behind the two Chinese characters "Hua Kan"——"Hua" is the "Hua" of "Tsinghua" and also the "Hua" of "China" (Zhong Hua), and "Kan" is taken from the "niche for a statue of Buddha" and has the meaning of "surrounding environment"; Its English logo directly uses the words "Cyto" and "Niche".
With such an original intention, CytoNiche has been running faster and faster on the road of cell "intelligent manufacturing" industry, leaping one step a year.
After receiving the Angel round investment in early 2019, CytoNiche started building a GMP plant. "I need to produce 3D microcarriers in the GMP plant, and then apply for qualifications." For this decision that seemed quite bold at the time, Liu Wei said so.
At the beginning of 2020, the company completed A round funding. The strategic investment of well-known funds, including international industrial groups, provided funds and help in formulating the future development strategies for the CytoNiche. In the same year, CytoNiche submitted the qualification application for pharmaceutical excipients of 3D microcarrier to CDE and FDA.

In 2021, CytoNiche first completed A+ round funding, and then 3D microcarrier obtained relevant qualifications from CDE and FDA, achieving "applications in both China and the United States". Among them, the qualification filing registration of FDA DMF pharmaceutical excipients (DMF: 35481) completed for 3D microcarrier is the only microcarrier product in the DMF record list published on the FDA official website.
In March 2022, CytoNiche announced the completion of nearly RMB 300 million raised in B round funding. In August, CytoNiche 3D Cell Intelligent Manufacturing Platform and Regeneration Medical Center —3D FloTrix®Cell CDMO Platform was officially unveiled.
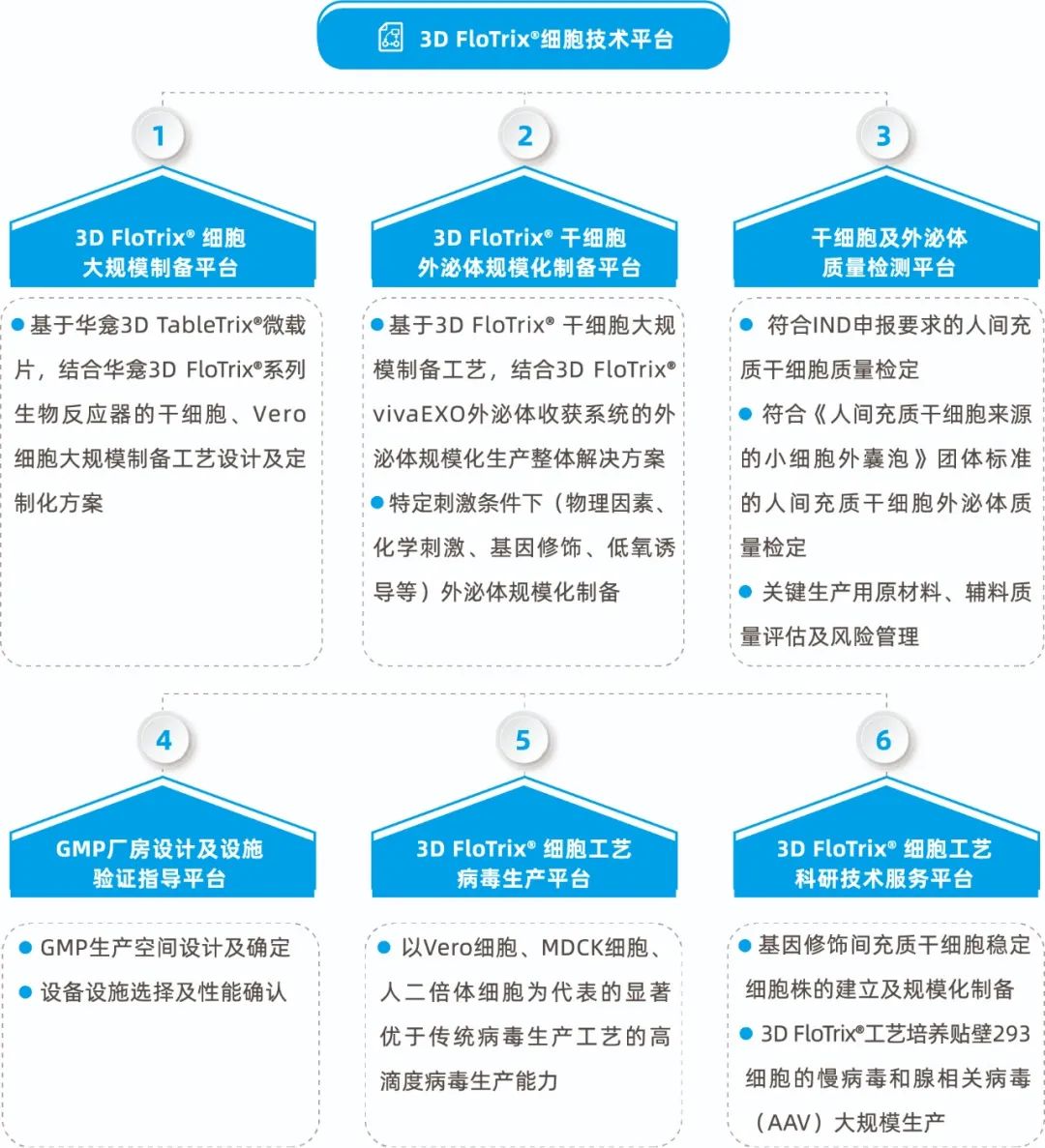
【CDMO services help reduce the cost of cell therapy】
In fact, before the establishment of CDMO platform, CytoNiche had already secured cooperation with a number of customers.
CytoNiche not only provides customers with a new technology, but also help them adapt related equipment systems and services to this new technology, to form a set of overall solutions. 3D microcarrier is a new technology different from the traditional two-dimensional culture. "Not every team can do a good job in 3D process. It takes time to explore." Therefore, it is natural to provide more services for customers and even develop CDMO business.
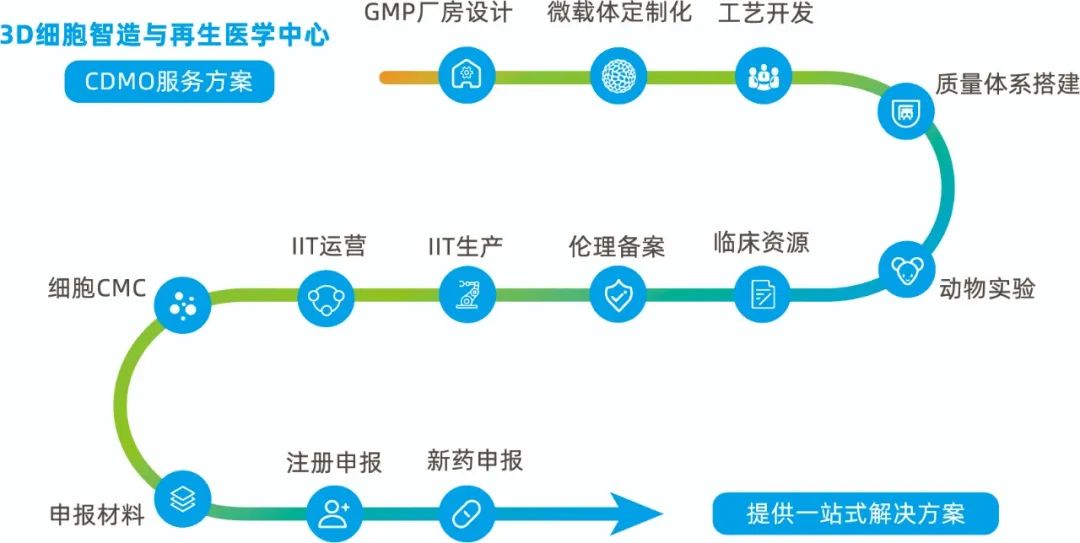
With the rise of cell gene therapy (CGT), many CDMO enterprises in China have turned attention to this field at present, relying on their previous experience in the fields of vaccines and antibodies. However, this transformation process also faces at least four challenges —— process, capacity, talent and cost. These challenges are common problems in the industry derived from cell gene therapy itself. Dr. Sun Yanxun, head of 3D FloTrix®Cell CDMO Platform, said.
Process comes first. There are many links involved in the production process of cell therapy products, whether immune cells or stem cells. Cell culture seems simple, but how to standardize and truly meet the quality requirements of cell medicine is a challenge. The traditional two-dimensional cell culture is a multi-batch, labor-intensive manual operation, which is difficult to ensure the uniformity of cell quality.
The second is production capacity. Nowadays, most of the enterprises engaged in the research and development of cell therapy products were transformed from scientific research-oriented enterprises. The cell production environment of most enterprises and institutions is still difficult to meet the production plant standard with GMP qualification. The capacity of traditional two-dimensional cell culture may meet the needs of phase I and II of clinical trials, but it falls short when it comes to phase III that involves large size of samples. However, with the transition from clinical trials to commercial production, the amplification of cell capacity and the requirements of GMP production conditions overwhelms the carrying capacity of smallest and medium-sized enterprises. At this point, they need to consider whether to enlarge the production scale or change the process.
The third is talent. As a new industry, most of the practitioners in cell therapy are previous scientific researchers. It takes times to turn their thinking to the perspective of the industry, and this process requires the support of practical experience. The traditional way of medicine quality management and control does not apply to cell therapy as a new "living" medicine Therefore, the talents of cell therapy products should have not only experience in cell process development, but also a deep awareness of regulatory policies and a clear understanding of the market demand for medicine development. This kind of multi-talents is the scarce resource in the current talent market.
Cost comes the last but not the least The challenges of process, production capacity and talents have led to the high cost of cell therapy products, which ultimately leads to the high market price of products and limits the application of cell therapy products. At present, patients may accept the high price of immune cell therapy products for blood tumor diseases because of the life-and-death situation involved. When the indication range of cell therapy is wider and wider, especially in some non-fatal cases, the high price is unsustainable. Only by reducing the production cost of cells, thus reducing the cost of cell therapy products and making them affordable to more patients, can cell therapy really have a market.
CytoNiche 3D microcarrier and its associated equipment can meet these needs. To produce tens of billions of cells the traditional two-dimensional cell culture method may require a lot of manpower and several months of culture time. In comparison, CytoNiche 3D culture system requires only 2 people and 10 days. The problems of traditional cell preparation methods, such as staffing requirement, complicated operation, large space energy consumption, and non-uniform quality across batches, can all be solved. "We provide CDMO services based on our own core technology and equipment to help customers solve various challenges of cell manufacturing and meet the market demand for lower cost reduction higher efficiency, so that cell therapy products can be affordable for the mass, instead of being the welfare for a few people."
It took about one year for 3D FloTrix® Cell Large-scale Production Process Overall Solution to be recognized by the industry. CytoNiche has also secured strategic cooperation with high-quality enterprises in the field of cell therapy such as Baylx Biotech and Topcel Kangheng. These customers have experienced 3D FloTrix®Cell Large-scale Production Process "from lab test verification, step-by-step amplification process, to quality verification and change of process". Taking Topcel Kangheng as an example, after many times of production verification, CytoNiche has helped them realize the production and quality inspection of 10 billion cells in a single batch in a short time, and the products meet the existing quality standards. Meanwhile, the production cost is reduced by 40%, the labor cost by 75% and the time cost by 50%.
【About the future...】
As for the future, CytoNiche will continue to expand the application of 3D microcarrier and other technologies. Apart from immune cells, stem cells, exosomes and cytokines and other cell medicines and their derivatives, 3D microcarrier can also be expanded to vaccines, antibodies, oncolytic viruses, gene editing and other virus and protein upstream processes, medical regeneration products and organoids, and even cell-cultured meat...
"Applying this technology to the field of vaccines can be described as a 'dimensionality reduction'." According to Liu Wei, there is no problem in using a vector that can be used to culture adherent cells for the amplification of ordinary virus. In fact, this is more efficient.
In addition, CytoNiche also plans to establish a "Southern R&D and Production base". At present, CytoNiche is headquartered in Beijing and its production is in Tianjin. Considering that domestic medicine innovation and research and development are more concentrated in Eastern and Southern China, in order to get close to more customers and bring product concepts, processes and services to them, he is considering to build a "Southern R&D and production base" in Eastern China.
As he has repeatedly stressed, it is not for "involution” in this field that CytoNiche engages in CDMO. In his plan, what CytoNiche really wants to do is to promote the industrialization of new cell large-scale culture technologies. Different from the CDMO business involved in the transformation or amplification of traditional medicine R&D enterprises, the CDMO business of CytoNiche is mainly based on 3D FloTrix®Cell Technology Platform System. Starting from the core original research technologies and products, CytoNiche tries to empower the whole CGT field, and also provides services of technical research and development and process development of new products of Chinese origin. This is the core competitiveness of CytoNiche's in entering the CDMO business. He even proposed to build a strategic cooperation alliance with more medicine R&D enterprises and the existing CDMO enterprises together to promote the technology development.
"We still have more 'long-term mechanisms'. Even if we don't do 'M (manufacturing)', there is still a long-term demand and market for consumables such as 3D microcarrier." He said with a smile.
[CytoNiche]
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
