Previously on the rising star of immunotherapy: NK cell therapy.
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2024-03-20
- Views:339
(Summary description)Growing NK cells, Huafan Biologicals has its own techniques.
Previously on the rising star of immunotherapy: NK cell therapy.
(Summary description)Growing NK cells, Huafan Biologicals has its own techniques.
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2024-03-20
- Views:339
The Development of Immunotherapy
In the past decade, there has been remarkable progress in cancer immunotherapy, which has become the mainstream method for treating various types of cancer. Immunotherapies based on T cells have been developed and successfully applied in clinical treatment for diverse cancers. However, the limitations of T cell immunotherapy, such as the risks of graft-versus-host disease (GVHD), cytokine release syndrome (CRS), and neurotoxicity, have become increasingly evident. Consequently, there is an urgent need to explore alternative anti-tumor effector cells and their immunotherapy strategies.
Natural killer (NK) cells are lymphocytes of the innate immune system capable of killing virus-infected and cancer cells. Unlike T cells, NK cells lack surface T cell receptors (TCRs), thus avoiding the induction of graft-versus-host disease (GVHD). The ability of NK cells to recognize and rapidly eliminate tumor cells, along with their limited reactivity to healthy tissues, suggests their potential as a novel immunotherapy. NK cells can be prepared, optimized, and administered to multiple patients on demand.
Over the past 20 years, NK cell-mediated immunotherapy has emerged as a safe and effective treatment for late-stage leukemia patients, with NK cell-based cancer therapy experiencing exponential growth. It has become a major area of innovative immunotherapy.
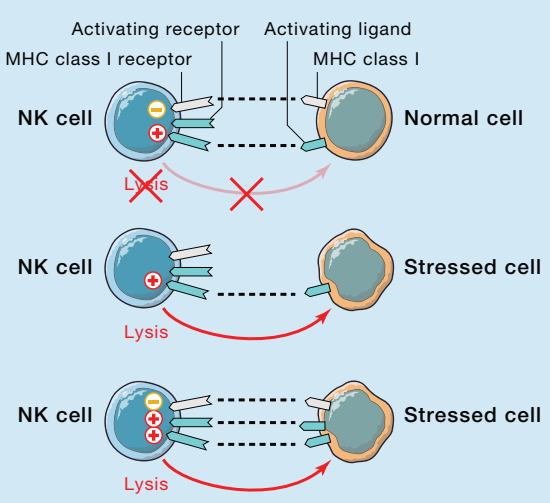
Figure 1: Regulation of NK Cell Function [4]
NK cell therapy widely used in clinics.
There are various sources of therapeutic NK cells currently undergoing clinical trials, including haploidentical NK cells, umbilical cord blood NK cells, stem cell-derived NK cells, and NK cell lines (Figure 2).

Figure 2: Sources and Expansion of NK Cells [5]
As of March 18, 2024, there are 11 NK cell drugs under review in China (Figure 3), with a total of 39 clinical trials of NK cell therapy in progress (Figure 4). These trials primarily target solid tumors, hematologic malignancies, and immune system disorders. Among them, there are 17 clinical trials for solid tumors, typically employing CAR and combination therapies to enhance targeting. There are 12 clinical trials for hematologic malignancies and 6 for immune deficiency-related diseases, as well as some clinical trials related to gynecological diseases and anti-aging, demonstrating a very broad application prospect.

Figure 3: Status of NK Cell Drug IND Acceptance
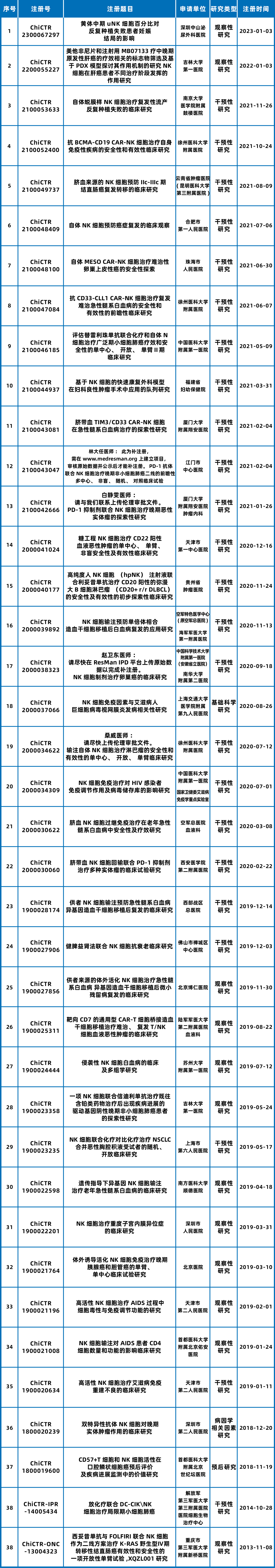
Figure 4: Clinical Trials of NK Cell Therapy
Challenges Facing NK Cell Therapy
Despite the great strides made in obtaining high-purity NK cells through the use of various strategies, including the feeder-free system, feeder cells, and some NK cell products that have already been tested in clinical trials, there are still challenges in the large-scale generation of NK cells.
Most NK cells used in clinical practice are produced in limited quantities and cannot meet the needs of all patients. Moreover, the large-scale production of NK cells typically requires 14 to 28 days, while the traditional manual production process necessitates frequent replenishment of fresh culture media, cytokines, and growth factors to ensure optimal growth conditions for the NK cells. This undoubtedly increases the risk of introducing external contaminants and requires a significant amount of manpower and financial resources.
Therefore, it is essential to develop a fully automated, fully enclosed system for the large-scale expansion of NK cells (Figure 5). Such a system not only ensures that the entire production process complies with GMP standards and improves the quality control of NK cell production but also can significantly save costs in the long run.

Figure 5: Large-scale expansion system for NK cells.
Disposable swirling culture system effectively expands NK cells
The disposable swirling culture system features a fully enclosed environment, single-use consumables, and a wave-like mixing method, which eliminates the need for frequent replacement of liquids or manipulation of cells, thereby greatly reducing the risk of contamination. Additionally, the unique wave-like rocking motion promotes oxygen fusion and reduces shear force. Compared to the traditional manual operation methods, the actively expanded NK cell yield under the swinging, fully enclosed, and automated culture conditions is higher.
Huahuan Biotechnology is about to launch a disposable swirling bioreactor——the 3D FloTrix® vivaROCK bioreactor system. This system relies on its unique stirring mode to provide excellent mixing effects and create a cell culture environment with low shear force and high dissolved oxygen, paired with self-developed disposable cell culture bags to form a fully enclosed culture system, which reduces the risk of contamination and improves the quality of cell expansion.
Expanding NK cells using the 3D FloTrix® vivaROCK bioreactor system: Inoculate pre-expanded NK cells into the cell culture bag at a cell concentration of 2×10^5 cells/mL, gradually add fresh serum-free culture medium until the total culture volume reaches 1.5L, and then initiate the perfusion culture.
NK Cell Experimental Data
Cell Information: NK-92
Inoculation Conditions: 2×10^5 cells/mL, 200 mL culture system
Equipment Parameters: Swing control at 6°, 6 rpm, culture temperature 37℃ + 0.2℃, filter membrane temperature 55℃, pH 7.2±0.05 (related to CO2), DO 50%±5%, total air intake 500 sccm, maximum intake of O2 and CO2 is 250 sccm each, pressure上限 31.0 mbar.

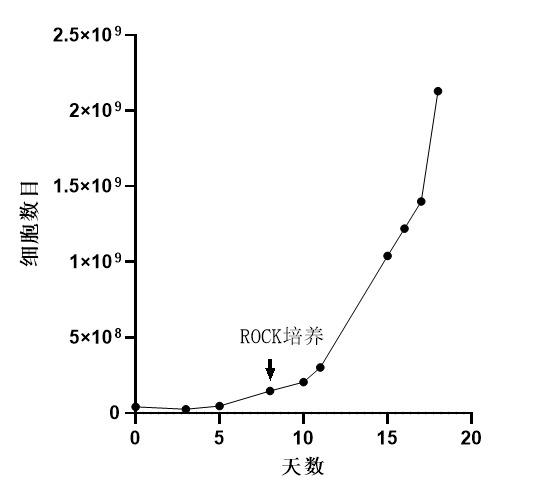
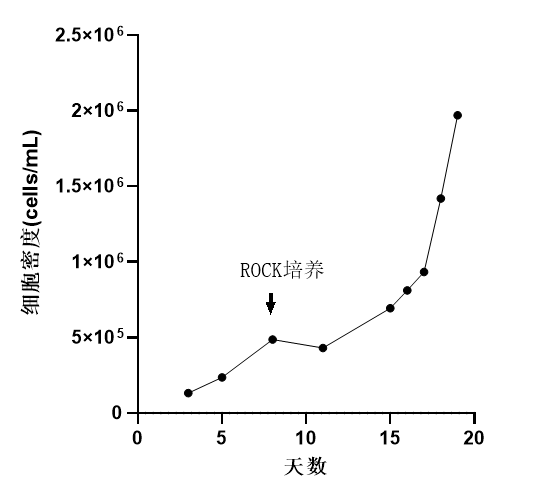


Cell Density: Reached 2.0×10^6 cells/mL, exceeding the literature's recommended culture density (1.1-1.3 x 10^6 cells/mL).
Cell Viability: Cell viability >70%.
Proliferation: Cells cultured for 11 days, resulting in a 20-fold increase in proliferation.
Cytotoxicity: When the effector-to-target ratio is 10:1, the killing rate of K562 cells is 75%.
References
[1] G. Sonpavde, PD-1 and PD-L1 Inhibitors as Salvage Therapy for Urothelial Carcinoma, The New England journal of medicine 376(11) (2017) 1073-1074.
[2] G. Xie, H. Dong, Y. Liang, J.D. Ham, R. Rizwan, J. Chen, CAR-NK cells: A promising cellular immunotherapy for cancer, EBioMedicine 59 (2020) 102975.
[3] N. Shimasaki, A. Jain, D. Campana, NK cells for cancer immunotherapy, Nature reviews. Drug discovery 19(3) (2020) 200-218.
[4] A. Crinier, E. Narni-Mancinelli, S. Ugolini, E. Vivier, SnapShot: Natural Killer Cells, Cell 180(6) (2020) 1280-1280.e1.
[5] Cellular & Molecular Immunology (2022) 19:460 – 481
[About CytoNiche]
CytoNiche was established in 2018, led by Professor Yanan Du's research team from the School of Medicine at Tsinghua University, with Tsinghua University as a shareholder. The core technology originated from the transformation of scientific achievements at Tsinghua University and was recognized as a leading technology in "Science and Technology Innovation in China" by the China Association for Science and Technology. As a national-level high-tech enterprise, a national-level specialized and new technology "Little Giant" enterprise, a potential unicorn enterprise, it has also received key research and development special support from the Ministry of Science and Technology.
As an expert in high-quality three-dimensional cell manufacturing, CytoNiche provides a one-stop customized solution for cell scale-up based on 3D microcarriers. The company has built an original 3D cell smart manufacturing platform, achieving large-scale, automated, intelligent, and closed-cell drug and derivative production preparation. This helps global customers establish the most advanced cell drug production lines. After pioneering the production process pipeline for "billion-level" stem cells, the company is accelerating towards "hundred billion-level," dedicating efforts to empower the cell and gene therapy industry with 3D cell scale-up smart manufacturing technology to benefit more patients.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
