CDMO of CytoNiche Facilitates Commercialization of Cell Therapy Products | GMP Production Environment of Cells
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-19
- Views:430
(Summary description)Different from most small molecule drugs and many biological drugs, the main forms of CGT products include gene therapy vectors, oncolytic virus products, cell products (such as CAR-T, NK cells, stem
CDMO of CytoNiche Facilitates Commercialization of Cell Therapy Products | GMP Production Environment of Cells
(Summary description)Different from most small molecule drugs and many biological drugs, the main forms of CGT products include gene therapy vectors, oncolytic virus products, cell products (such as CAR-T, NK cells, stem
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-19
- Views:430
Different from most small molecule drugs and many biological drugs, the main forms of CGT products include gene therapy vectors, oncolytic virus products, cell products (such as CAR-T, NK cells, stem cells), etc. The upstream process development services involved can be divided into three relatively independent links including plasmid, virus and cell production process.
Therefore, CGT products are usually produced in the unique manufacturing environment, and their GMP plants shall be designed and built according to the characteristics of their respective processes. Also, they shall adapt to the changing process improvement and technical requirements in the future, so as to meet not only the current commercial GMP production capacity requirements but also the needs in the future.
Due to the characteristics of significant individual differences, inability to disinfect and sterilize the end products, and long production cycle, cell therapy products can only rely on process control to ensure the safety of their end products. The air cleanliness level of production environment and facilities shall adapt to the products and production operations. The production environment and facilities shall not cause contamination to the raw materials, intermediates and end products.
This article only discusses the design of process plane and plant facilities involved in MSCs product process, and how to make large-scale preparation to meet the needs of GMP commercial manufacturing.
MSCs therapy products shall follow GMP guidelines and produce drugs according to IND laws and regulations and quality requirements. GMP plant design is the basis to produce MSCs products, and applied to ensure the most advanced quality standards suitable for clinical use applied to the continuous production and control of MSCs products. The production process flow of MSCs cell therapy products is mainly divided into the following stages:
▮Supplier selection and evaluation (allogeneic MSCs products).
▮Receiving and separating tissue samples.
▮Preparation of primary culture cells.
▮Establishment of seed cell bank.
▮In vitro cell amplification to obtain the expected number of cells.
▮Establish working cell bank for intermediate products.
▮Preparation, identification, packaging and release of final preparation products.

Accordingly, a GMP production workshop can be divided into the following functional areas, on the example of CytoNiche 3D Cell Intelligent Manufacturing and Regeneration Medical Center——3D FloTrix® Cell Technology Platform:
1. Receiving area for samples and materials:
This area is used to receive and register the tissue samples collected by medical institutions and found to meet the receiving and registration standards in testing, and deliver them to the buffer zone. The buffer zone is mainly used for the preliminary treatment of tissue samples and the temporary storage of raw and auxiliary materials. Refrigerators shall be in place to meet the requirements of temperature on the suppliers' materials and product quality. The cleanliness level C is enough.
2. Cell operation area:
At present, the cleanliness level of the production and operation environment of cell therapy products can be divided into the following levels according to Good Manufacturing Practice of Medical Products-Appendix of Cell Therapy Products:
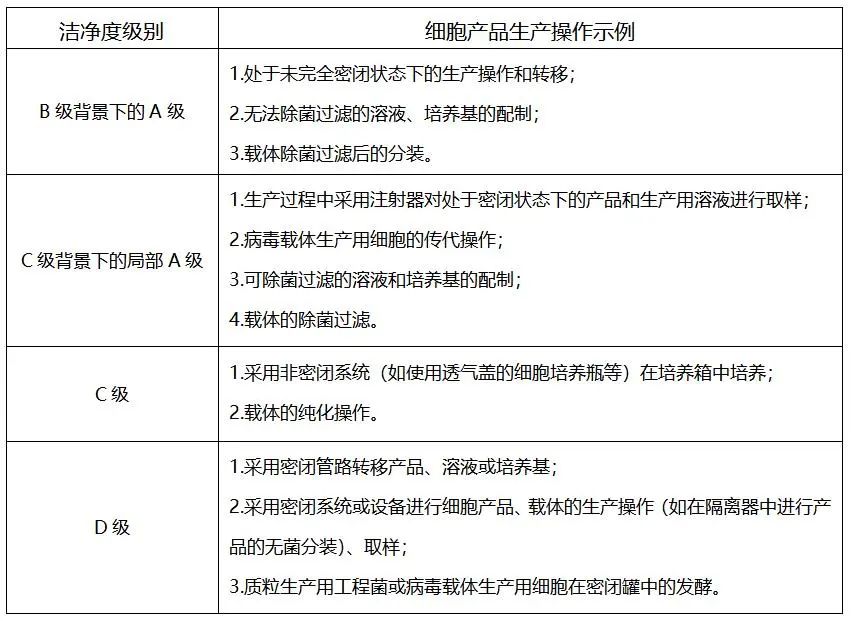
At present, the cleanliness level of MSCs therapy products commonly applied in the cell therapy enterprises can be divided into B+A and C+A. For example, if the traditional two-dimensional adherent cell culture approach is adopted, the B+A form is the first choice to realize the production environment, and the cleanliness level of the room is class B. Since there are non-fully-enclosed operations such as culture flasks/cell factories, it is necessary to carry out cell culture in the biosafety cabinet under the condition of class A, and then in the CO2 incubator. However, this method cannot guarantee that the whole process can be completed in a class A environment. With the development of the production equipment of cell therapy products towards full closure and automation and the application of the sterility isolator, the requirements for the production environment will also be reduced. The cleanliness level can also be downgraded from class B to class C or D. The key links such as centrifugation and culture can be integrated in the isolator to achieve class A isolation throughout the process.




Live scene of CytoNiche 3D FloTrix®Cell Technology Platform
The cell operation area of CytoNiche is mainly used for the production and operation of MSCs cell products from different tissues. The area is divided into primary culture cell separation and preparation room, cell amplification culture room and cell preparation room. The cleanliness level of the production environment is divided into B+A and C+A throughout the process according to the characteristics of the two-dimensional and three-dimensional culture processes, which can meet the needs of different customers for the cleanliness level. CytoNiche 3D FloTrix®vivaSPIN Bioreactor is a closed, automated and large-scale stirred bioreactor independently developed by CytoNiche. Combined with CytoNiche 3D FloTrix®vivaPREP Cell Harvesting System, according to the specific cell types and process requirements, it can realize a continuous and closed production process from cell seeding, culture and amplification, cell harvesting and packaging in a class C environment, improve the single-batch cell yield, significantly reduce the production cost and reduce the risk of quality instability across batches caused by repeated cell preparation.




Live scene of CytoNiche 3D FloTrix®Cell Technology Platform
3. Process auxiliary area:
In the design of GMP functional areas, full consideration should be given to that the positioning and maintenance of facilities such as clean gas, pure water, power supply, medical waste disposal, cleaning, disinfection and sterilization, purified air conditioning system, environmental monitoring system, etc., to satisfy their expected purposes and uses and ensure that the cleaning, disinfection, maintenance and monitoring operations of the production environment and facilities of MSCs products are regularly verified and continuously controlled.
▮MSCs products cannot be finally sterilized or filtered for sterilization. If the dosage is not large in the process, purchased sterile water and reagents can be used.
▮Purified water shall be used for the cleaning of the clean area, including work clothes and production equipment.
▮It is required to provide UPS power to key equipment, such as cell separation and amplification equipment, CO2 incubator, refrigerator, key analytical instruments, etc.
▮The production equipment and medical wastes in and out of the B-class area shall be sterilized in the humid heat sterilization cabinet.
▮The pressure difference between clean areas and non-clean areas, and between clean areas of different cleanliness levels shall be no less than 10 Pa.
▮3D FloTrix®process gas involves the use of air, oxygen, nitrogen and carbon dioxide. The gas pipeline is designed with one-way flow. The integrity of the terminal sterilization and filtration device is regularly checked.
▮The cell operation room of CytoNiche is designed with a one-way flow. The exit channel of wastes is separated from the clean channel of products and production materials, and an independent waste corridor and waste inactivation room are set up to minimize cross contamination.
4. Quality control area and storage area:
CytoNiche quality control area includes cell culture testing room, physical and chemical testing room, molecular testing room, positive control room, sterility testing room, etc., involving mycoplasma, endotoxin, sterility, microbial limit, positive control, flow cytometer, molecular biology and other related tests, which can meet the requirements of all quality inspection items of MSCs cell therapy products. The storage area includes the storage of all levels of cell banks, reagents, and consumables. The liquid nitrogen storage area shall take the load bearing capacity of the floor into full consideration.
The characteristics of cell therapy products determine the complexity of process flow design and the special requirements of the auxiliary system. In the process layout and plant facilities of its production workshop, factors such as the site selection, production varieties and batches, process, product R&D and production stage should be fully considered. Only reasonable and compliant plant design can ensure the safety and legality of products.
CytoNiche 3D Intelligent Manufacturing and Regeneration Medical Center has established a complete GMP quality management system, covering production management, quality control and quality assurance, plants and facilities, materials and products, confirmation and verification, entrusted production and entrusted verification, product shipment and recall, self-inspection, etc.

All production and quality control personnel have received systematic training and they carry out production, recording, and inspection in strict accordance with the requirements of quality management system, thus realizing the traceability management of the whole production process and meeting the needs of pre-clinical CDMO process development and services of stem cell therapy products.
For reference:
1. 2022 Good Manufacturing Practice of Medical Products——Appendix of Cell Therapy Products
Coming up next: Equipment and Consumables for 3D Cell Culture
For details, welcome to follow CytoNiche.
[CytoNiche]
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
