CDMO of CytoNiche Facilitates Commercialization of Cell Therapy Products | Equipment and Consumables for 3D Cell Culture
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-24
- Views:590
(Summary description)In the field of stem cell therapy products, at present, cell culture mainly adopts the open production process based on cell culture flasks and multi-layer cell factories. Some enterprises adopt isola
CDMO of CytoNiche Facilitates Commercialization of Cell Therapy Products | Equipment and Consumables for 3D Cell Culture
(Summary description)In the field of stem cell therapy products, at present, cell culture mainly adopts the open production process based on cell culture flasks and multi-layer cell factories. Some enterprises adopt isola
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-24
- Views:590
In the field of stem cell therapy products, at present, cell culture mainly adopts the open production process based on cell culture flasks and multi-layer cell factories. Some enterprises adopt isolators combined with honeycomb incubators to realize the closed stem cell culture process, but it is still a manual operation, and difficult to realize the automatic, large-scale and standardized stem cell culture process.
The application of bioreactors for large-scale production and preparation of stem cells not only meets the demand for cell yield in the process of clinical application and commercialization of stem cell products, but also avoids the contamination risk caused by the open operation, and significantly reduces the cost of consumables, manpower and time in the process of cell production and preparation. In addition, harvesting a large number of cells at one time can better ensure the quality uniformity of the end products of cells and reduce the differences across batches in the process of cell preparation.
However, no matter what bioreactors and microcarriers are adopted for the large-scale preparation of stem cells, we must not blindly pursue cell amplification multiples times and cell yield. Any large-scale preparation process of stem cells shall take the key quality attribute of cells and the stability of the process as the evaluation criteria.
The selection of different scales of cell preparation process of stem cells has been elaborated in detail in the previous article "Large-scale Production Process and Quality Control of 3D Microcarriers of Stem Cells (Part 1 and Part 2)", and won't be repeatedly described here.
Click the picture to read the previous article.

For large-scale and commercialized production, the automatic closed cell amplification and harvesting systems are the development trend of the production process of cell therapy products. Article 11 of 2021 Good Manufacturing Practice of Medical Products-Appendix of Cell Therapy Products stipulates that "closed systems or equipment should be adopted for the production and operation of cell products, and the cleanliness class of the environment where the closed systems or equipment are placed can be appropriately reduced."
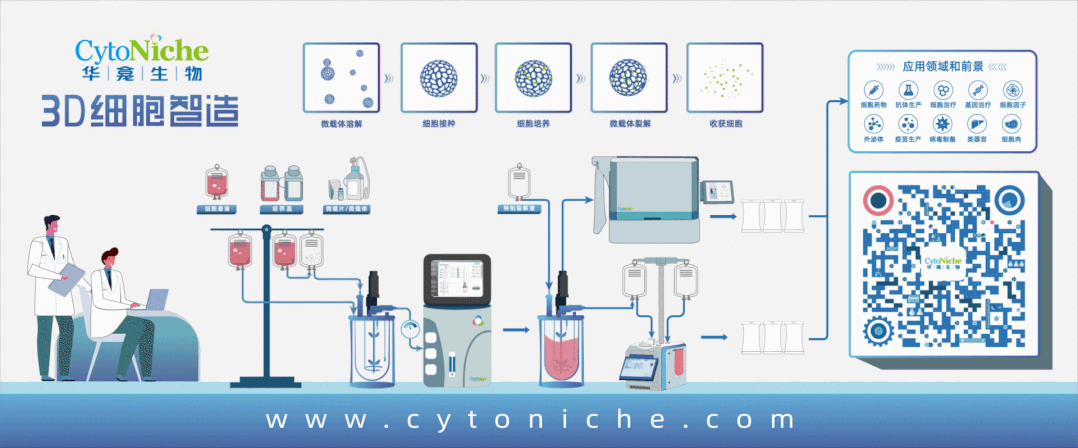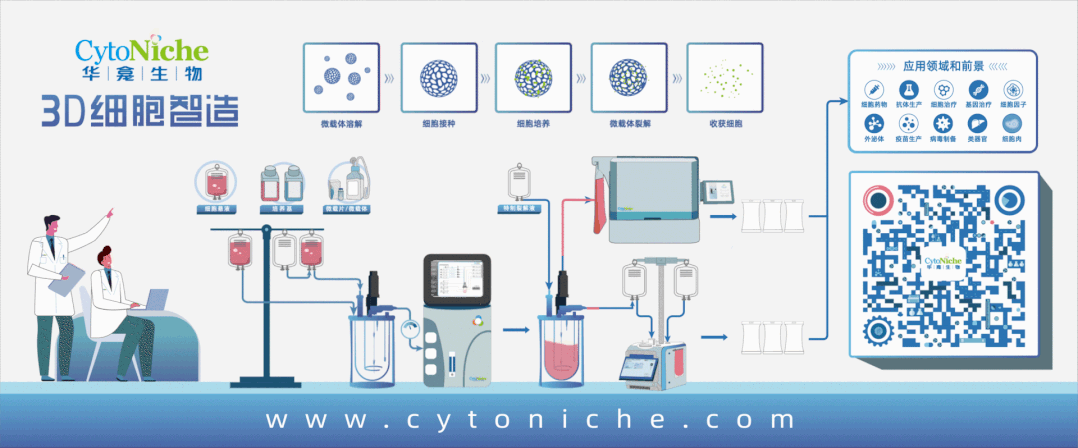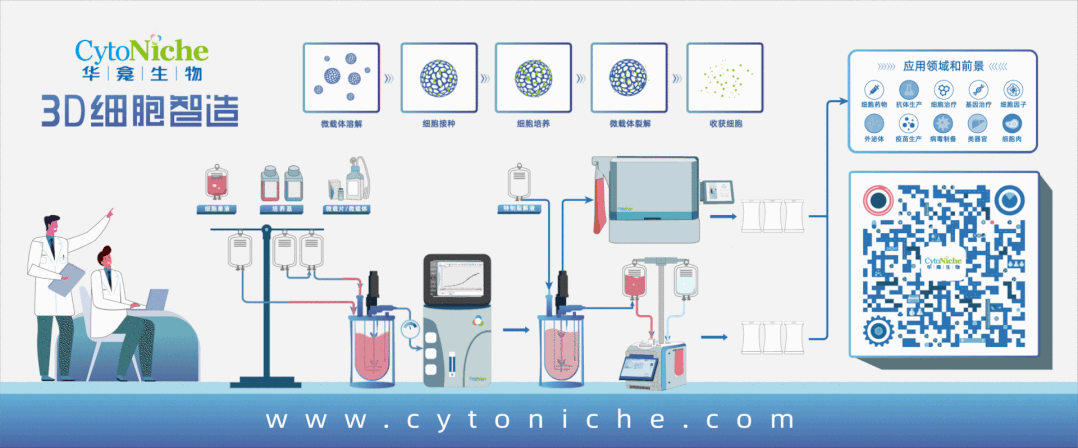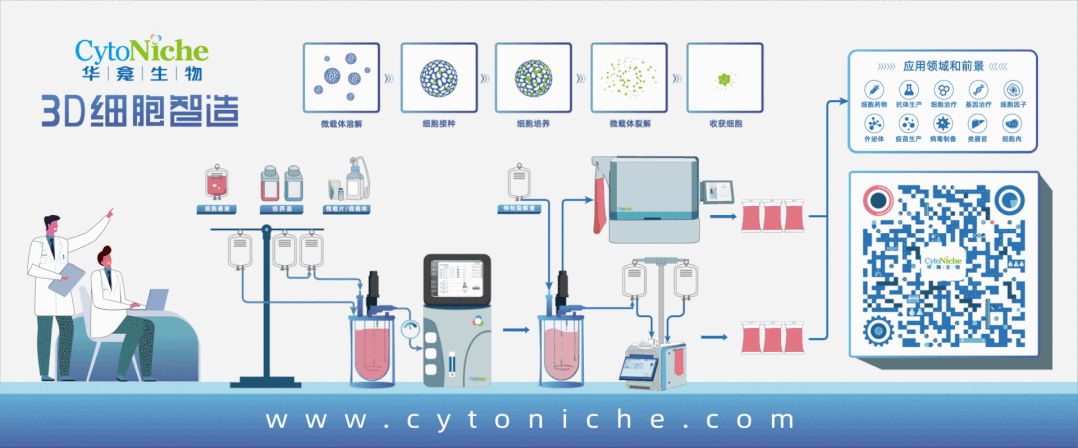
3D FloTrix® Automated Bioreactor is a closed, automatic and standardized stirred bioreactor independently developed by CytoNiche to realize the large-scale culture of 3D cells. At present, this series of products includes 125ml, 2L, 5L and 15L systems to meet the needs of different production processes and production capacities.
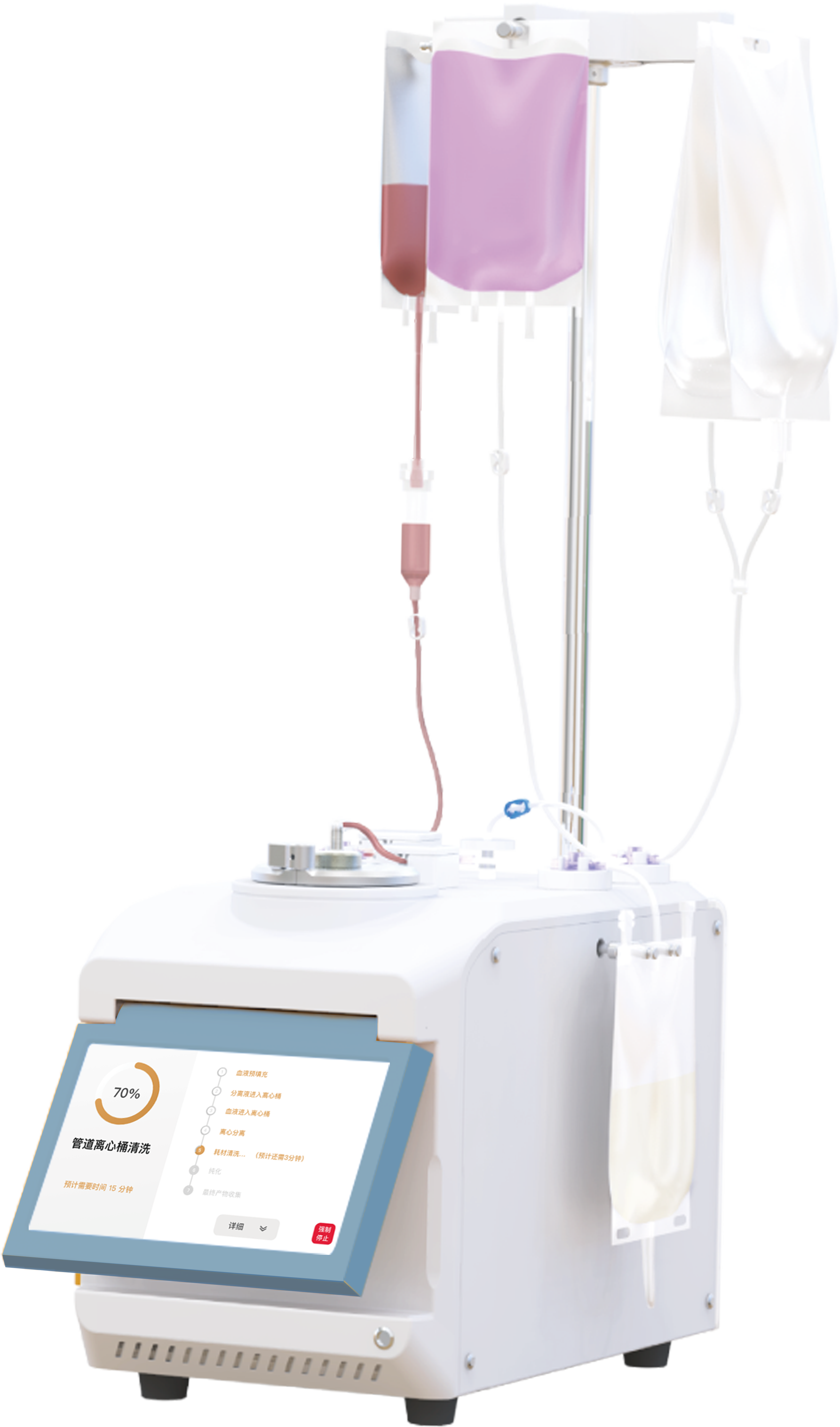
Based on the core technology product 3D TableTrix® Cell Microcarrier and 3D FloTrix® Dynamic Culture Process, 3D FloTrix® Cell Technology Platform combined with CytoNiche 3D FloTrix® vivaPREP Cell Harvesting System has the following advantages in the stem cell CMC process:
1. According to the specific cell types and process requirements, continuous and closed production process from cell seeding, culture and amplification, cell harvesting and packaging can be realized in a C-class environment, which can improve the yield of single batch of cells, significantly reduce the production cost, and reduce the risk of quality instability across batches caused by repeated cell preparation.
2. According to Article 36 of Good Manufacturing Practice of Medical Products-Appendix of Cell Therapy Products", the adoption of cell culture in the closed system allows the culture and the storage of different batches of products in the same production operation room or the same incubator simultaneously". The adoption of CytoNiche 3D FloTrix®Dynamic Culture Process allows the amplification and culture of multiple batches of cells at the same time in the same production operation room, which can ensure low risk of contamination and efficient use of clean space.
3. 3D FloTrix®vivaSPIN Automated Bioreactor Equipment, equipped with 3D FloTrix®CELL PRO Living Cell Monitoring System, can accurately monitor the status of living cells in real time and automatically generate cell proliferation curves. Equipped with the PECALSTM Control System, the equipment can also accurately execute automatic programs, support remote parameter control, and record all information in real time. Users can set user permissions and process parameters according to their own needs, and the cell data is complete and traceable.


The adoption of suspended bioreactor for the large-scale amplification of stem cells result in that stem cells usually adhere to the microcarriers suspended in the stirred vessel. As the adherent culture medium of stem cells, microcarrier is the key raw material applied in the production of stem cell products, with direct effects on the purity, quality and therapeutic effect of cells. Therefore, the selection of appropriate microcarrier is the key variable to ensure the quantity and quality of cells during stem cell culture and amplification.
2021 Technical Guidance for the Pharmaceutical Research and Evaluation of Human-Derived Stem Cell Products (Exposure Draft) also clearly points out that "some raw materials are in the process of continuous updating and optimization, and priority shall be given to the raw materials of low risk, such as pharmaceutical-grade raw materials"; "For all raw materials that may affect the product quality and safety, it is necessary to evaluate and control the residues at a reasonable stage of the final product or process, and the residue limit to be controlled shall be determined in combination with the clinical dosage".
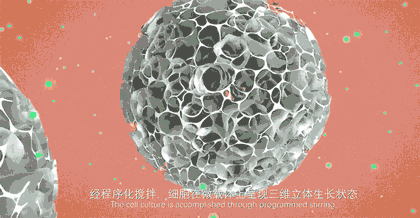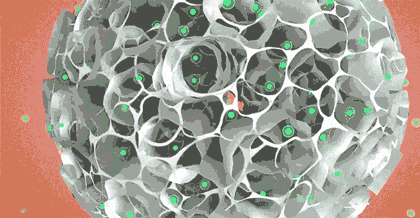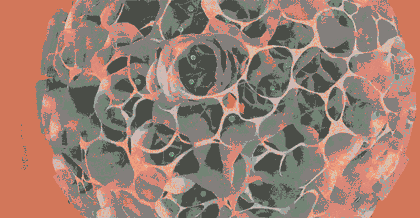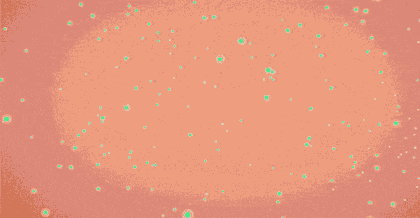
1: CytoNiche 3D TableTrix® Microcarrier
CytoNiche 3D Gelatine Microcarrier (3D TableTrix®) is a porous microcarrier with its core component being animal-derived protein and cross-linking agent that are solidified into the porous microsphere with 100-200µm diameters. The raw materials of microcarriers are pharmaceutical excipients. 3D TableTrix® Microcarrier, has passed the quality inspection and safety evaluation by a third-party, Jiangsu Center for Safety Evaluation of Drugs, as detailed in the "Quality Inspection and Safety Evaluation Report of Microcarrier". On this basis, it has obtained the qualification for pharmaceutical excipients from CDE of National Medical Products Administration (F20210000003 and F20200000496), and also from FDA (DMF: 35481).
2: CytoNiche 3D FloTrix®Digest Microcarrier Lysis Buffer
In order to remove the microcarrier after the cells amplified by the microcarrier reach the expected proliferation number, harvest the cells while ensuring the safety of cell therapy products and that the harvested cells meet the basic biological quality requirements of stem cells, CytoNiche developed the microcarrier lysis buffer (3DFloTrix® Digest Microcarrier Lysis Buffer) that can specifically degrade 3D TableTrix®Microcarrier and degrade the microcarrier into amino acid fragments. In order to ensure that the products after degradation (microcarrier residues and residues of lysis buffer) meet the safety standards of cell products in the final stem cell suspension, quality control is carried out from three aspects: The selection of raw materials, the establishment of inspection methods and safety evaluation. In addition, CytoNiche jointly establishes the residue detection methods and standards for detecting the specific amino acids in the microcarrier raw materials and enzyme activities in the lysis solution with National Institute for Food and Drug Control, and obtains "Quality Evaluation Report of Microcarrier Residues and Residues of Lysis Buffer" (National Institute for Food and Drug Control).

In the process of CGT products transiting from clinical trials to commercial manufacturing, how to develop and optimize the process is a challenge for both CGT development enterprises and CDMO service enterprises. We have discussed this in the previous article "The Application of Large-scale Preparation of Cells Based on Degradable Microcarrier in CMS Upstream Process Development".
Click the picture to read the previous article.
In this process, by selecting appropriate automation equipment, consumables and cell CMC process, the open manual process for clinical production will be expanded to the commercialized large-scale production, which not only meets the commercial demand of cell production, but also significantly reduces the production cost of cell therapy products and ensures the quality and safety of cell therapy products. CGT product developers are required to provide forward-looking commercial design, adopt breakthrough technologies and new automated solutions to meet the future development trend of cell and gene therapy.
For reference:
1. Technical Guidelines for Pharmaceutical Research and Evaluation of Human-derived Stem Cell Products (Exposure Draft)
2. Technical Guidelines for the Research and Evaluation of Cell Therapy Products (Trial)
3. Large-scale Production Process and Quality Control of 3D Microcarriers of Stem Cells (Part 1 and Part 2): CytoNiche public account/Stemceller public account
4. The Application of Large-scale Preparation of Cells Based on Degradable Microcarrier in CMS Upstream Process Development: CytoNiche public account/Stemceller public account
Coming up next: Stem Cell Therapy Products and Services
For details, welcome to follow CytoNiche.
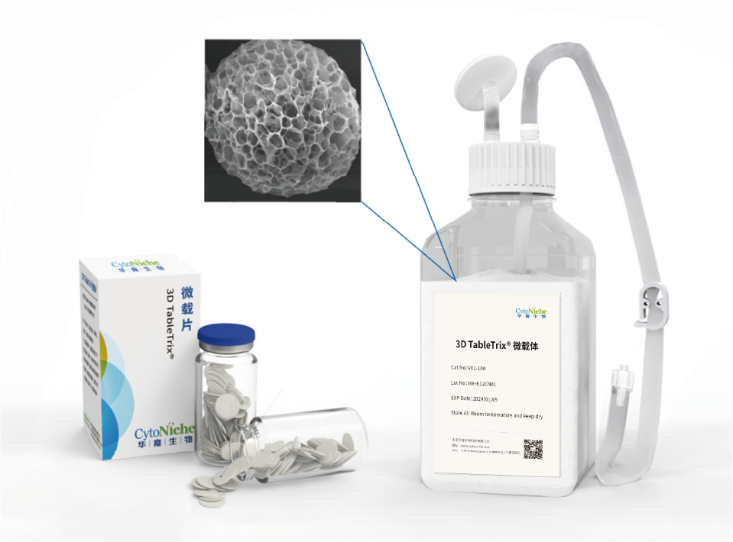
✦ 3D TableTrix®
Cell microcarrier
• Has obtained the qualification for pharmaceutical excipients from FDA (DMF) and CDE.
• Elastic porous structure with large specific surface area
• 3D biomimetic, customizable
• Specific degradation technology
• Aseptic tablet packaging, no need for pretreatment

✦ 3D FloTrix®
Three-dimensional stem cell culture medium
• Customized for 3D mesenchymal stem cell culture process
• GMP-grade production
• Serum-free, animal component-free
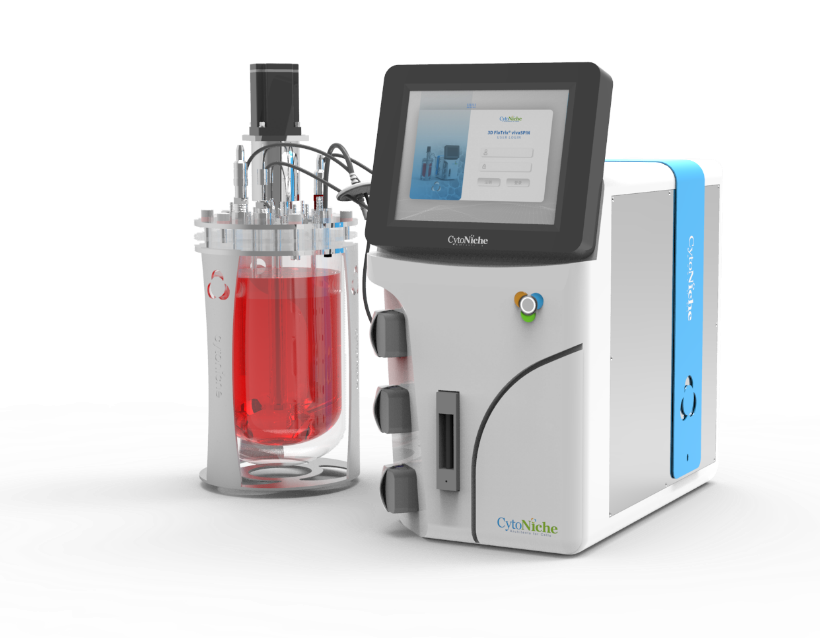
✦ 3D FloTrix®vivaSPIN
Automated bioreactor
• Implementation of 3D cell real-time online counting
• High precision mass flowmeter, enabling reduction in bubble generation, suitable for 3D cell dynamic culture process
• 3D TableTrix® cell microcarrier dedicated supernatant filtration system
• Audit trail function available, providing 3Q certification and complying with GMP
✦ 3D FloTrix®vivaSPIN
Automated bioreactor
• Implementation of 3D cell real-time online counting
• High precision mass flowmeter, enabling reduction in bubble generation, suitable for 3D cell dynamic culture process
• 3D TableTrix® cell microcarrier dedicated supernatant filtration system
• Audit trail function available, providing 3Q certification and complying with GMP

✦ 3D FloTrix®vivaEXO
Exosome harvesting device
• Capable of concentrating 10L of stock solution into less than 500mL in one single batch in 1-3 hours
• Adopting disposable fully enclosed consumable packs that are ready out of the box
• Supports setup and display of various parameters in the running process in real time.
[CytoNiche]
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP




 京公网安备 11010802037749号
京公网安备 11010802037749号
