-
 December 2023
December 2023Exciting News | CytoNiche Biotech Honored on Deloitte's "China Pharmaceutical and Health Tomorrow Star" List.
-
 November 2023
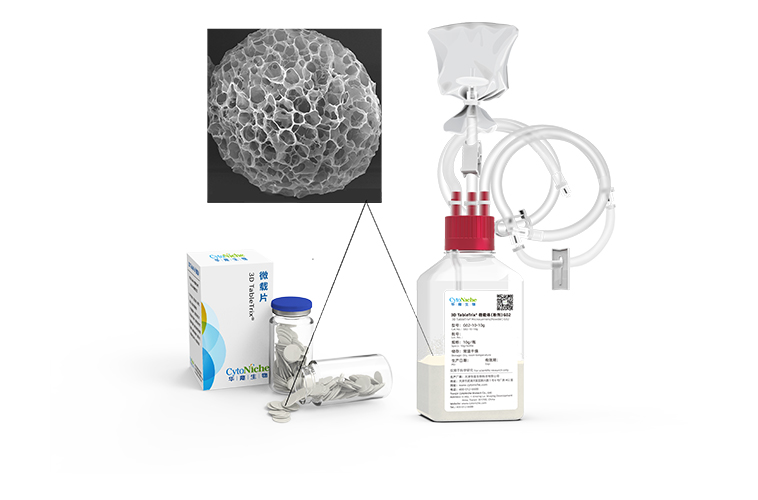
November 20233D TableTrix® G Series microcarrier, Focused on the gene therapy.
-
 November 2023
November 2023The 3D FloTrix® vivaFILL cell filling system is dedicated to the filling of "live" cell preparations. It focuses on various cell formulations and extracellular vesicle formulations, ensuring the effic
-
 November 2023
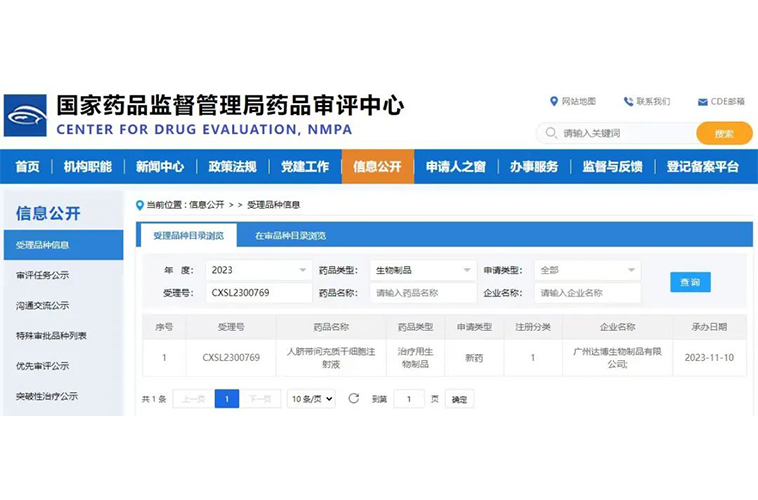
November 2023The world's first stem cell-based new drug, utilizing the Cytoniche's 3D FloTrix® process, has been accepted by the China Drug Evaluation (CDE).
-
 September 2023
September 2023As a key unit of the association, Cytoniche actively participated in the drafting of the "Quality Management Specification for Raw Materials Used in the Production of Cell Therapy Products" (Group Sta
-
 September 2023
September 2023Cytoniche's【3D FloTrix® Billion-Scale Process】 for the preparation of human umbilical cord mesenchymal stem cells has once again received authoritative third-party quality certification (Number: Inspe
-
 September 2023
September 2023Cytoniche's 3D microcarrier achieves authoritative certification from the China National Institute for Food and Drug Control (NIFDC) for the fully closed-scale preparation of human umbilical cord mese
-
 August 2023
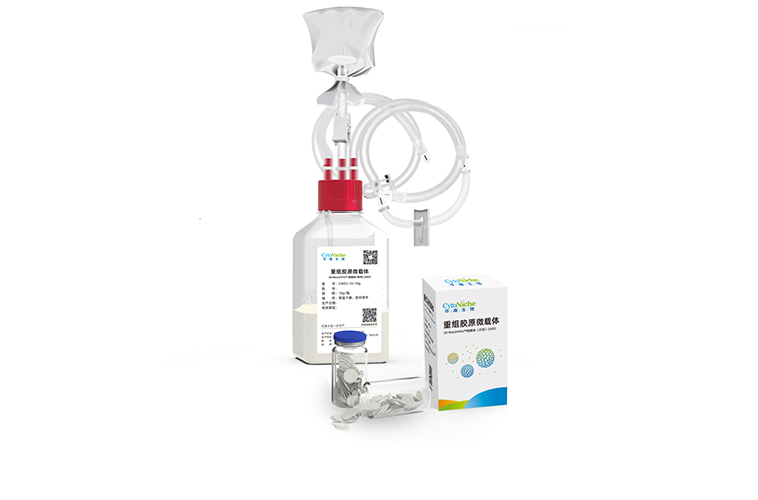
August 2023The 3D RecomTrix® recombinant collagen microcarrier retains the core features of the 3D TableTrix® microcarrier. The unique difference lies in the fact that it is composed of non-animal sourced recomb
-
 July 2023
July 2023The 3D TableTrix® V Series microcarrier ,Focused on the vaccine field.
-
 July 2023
July 20233D TableTrix® Microcarriers developed by CytoNiche has been filed with FDA-MF (MF: 29721)
-
 July 2023
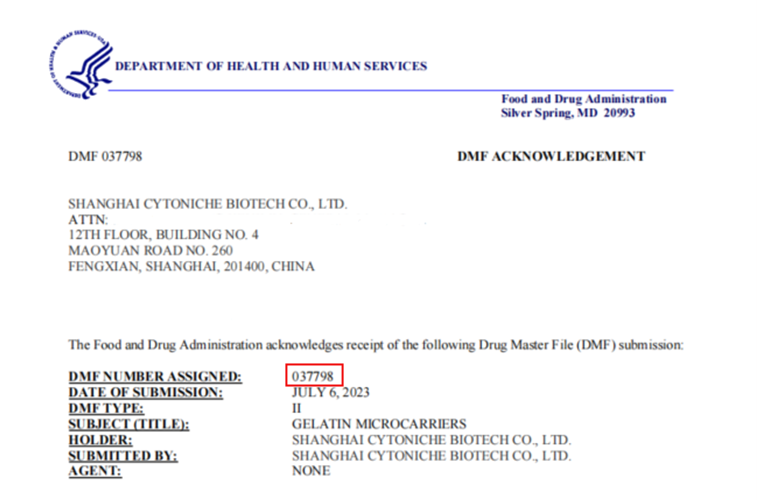
July 20233D TableTrix® Microcarriers developed by CytoNiche has been filed with FDA-DMF (DMF: 037798)
-
 June 2023
June 20233D FloTrix® mesenchymal stem cell serum-free medium developed by CytoNiche has been filed with FDA DMF (DMF: 038476).
-
 May 2023
May 2023CytoNiche made the list of the "2023 Future Healthcare 100" series
-
 May 2023
May 2023CytoNiche International Center for Cooperation and Technology Application was established
-
 May 2023
May 20233D FloTrix®vivaPACK Cell Filling System effectively completes the packaging of cell preparations, enabling the filling process of more than 100 bags of various cell fully enclosed preparations
-
 March 2023
March 20233D FloTrix®vivaPREP PLUS Cell Harvesting System provides efficient cell production and preparation to support the cell therapy industry
-
 February 2023
February 2023CytoNiche has been honored on the "Science and Technology in China" Pilot Technology List by the China Association for Science and Technology
-
 February 2023
February 2023CytoNiche makes the top 100 list
-
 December 2022
December 2022CytoNiche selected as 2021 GEI China Potential Unicorn
-
 November 2022
November 2022Approved to set up a postdoctoral scientific research workstation
-
 August 2022
August 20223D FloTrix® Cell CDMO platform was officially unveiled, and the expansion of the Beijing headquarters was completed
-
 August 2022
August 2022Topped the list of medical enterprises with 10 times growth potential of 36 krypton
-
 June 2022
June 2022Listed in KPMG China's first Biotechnology Innovation 50 Enterprise List
-
 April 2022
April 2022Tsinghua University & Huakan Biology's original research technology won the Geneva Invention Gold Medal
-
 March of the same year
March of the same year3D FloTrix® Microcarriers for Vaccines Launched
-
 March 2022
March 2022Completed nearly 300 million yuan in round B financing, accelerating the industrialization of 3D cell "smart manufacturing"
-
 February 2022
February 2022Tianjin GMP production platform expanded to 4000 square meters, new 1200L microcarrier production line
-
 July 2021
July 2021The cumulative annual sales exceeded 10 million yuan, and the financing of nearly 100 million yuan was completed
-
 June 2021
June 2021The GMP manufacturing platform was successfully certified by the ISO9001 and IS013485 quality management systems
-
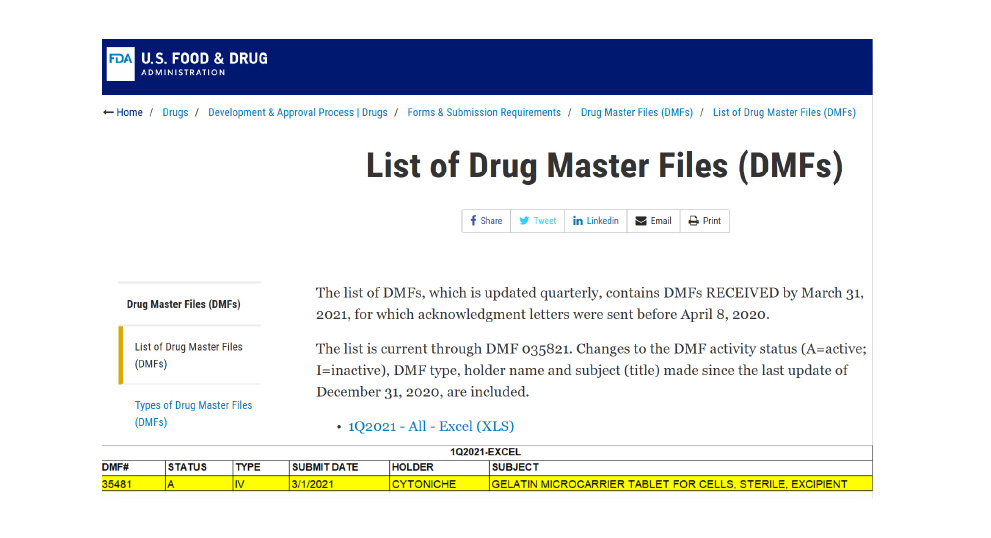 April 2021
April 20213D TableTrix® Cell Microcarrier Tablet obtained the qualifications DMF for pharmaceutical excipients from the US FDA (DMF:35481)
-
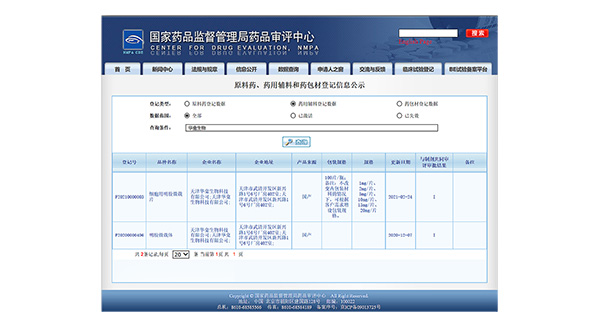 February 2021
February 20213D TableTrix® Cell Microcarrier Tablet obtained two qualifications for pharmaceutical excipients from CDE (Filing No.:F20200000496/F20210000003)
-
 December 2020
December 20203D TableTrix® microcarrier core products were released in United States, the European Union, Japan and other countries
-
 October 2020
October 20203D FIoTrix@ VS series of pilot-scale production-grade bioreactors were officially released in the market
-
 June 2020
June 2020Proprietary products of 3D TableTrix® series of microcarrier tablet officially were officially released in the market
-
 January 2020
January 2020The certification of intellectual property management system was completed
-
 October 2019
October 2019followed by A GMP-certified manufacturing base of 2000 square meters launched in the next month.
-
 December 2019
December 2019The application for "Zhongguancun Subversive Project" was successful; the certification of "National High-tech Enterprise" was obtained and the company ranked among top 3 in "Global Entrepreneurship World Cup" at the same time
-
 March 2019
March 2019In March 2019, Cytoniche received angel fund and a R&D center of 1500 square meters was therefore launched.
-
 2018
2018In 2018, the technology was successfully translated into Beijing CytoNiche Biotechnology Co., Ltd., joined by Tsinghua University equity-wise.
-

After more than 10 years of research in the field of cell microcarriers, Professor Du Yanan of Tsinghua University along with his research team have published a number of international leading research achievements internationally
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP












































 京公网安备 11010802037749号
京公网安备 11010802037749号
