CDMO of CytoNiche Facilitates Commercialization of Cell Therapy Products | Overview
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-17
- Views:0
(Summary description)In recent years, the global biologic drug market is under rapid development, with a growth rate significantly higher than the overall pharmaceutical market, especially in the field of the cell and gen
CDMO of CytoNiche Facilitates Commercialization of Cell Therapy Products | Overview
(Summary description)In recent years, the global biologic drug market is under rapid development, with a growth rate significantly higher than the overall pharmaceutical market, especially in the field of the cell and gen
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-17
- Views:0
In recent years, the global biologic drug market is under rapid development, with a growth rate significantly higher than the overall pharmaceutical market, especially in the field of the cell and gene therapy (CGT). Driven by demands for addressing various indications, a variety of CAR-T therapies, stem cell therapies, and gene therapies based on adeno-associated virus and lentivirus have emerged, providing important treatment options for relapsing and refractory tumors, and severe genetic defects. Among these, cell therapies based on unique action mechanism can provide new treatment options for refractory diseases for which traditional therapies prove ineffective. At present, cell therapies are divided into immune cell therapy, stem cell therapy and other somatic therapies.
In 2021, with the approval of Fosun Kite's axicabtagene ciloleucel injection and JW Therapeutics’ relmacabtagene autoleucel injection, China has ushered in two commercial CAR-T products, marking the official onset of China’s CGT drug market. Although no stem cell products have been approved for sale in China, as of July 2022, a total of 37 stem cell products from 28 companies have been accepted for clinical trial applications in China, and 26 products from 21 companies have been approved for clinical trials through Investigational New Drug (IND) applications, with indications involved including inflammatory enteritis, idiopathic pulmonary fibrosis, refractory acute graft-versus-host disease (GVHD), rheumatoid arthritis, knee osteoarthritis, chronic periodontitis, ischemic stroke, diabetic foot ulcers, COVID-19 caused acute respiratory distress syndrome, liver failure, anal fistula, and acute respiratory distress syndrome.
【Challenges faced by cell therapy products】
As cell therapy enters the age of commercialization in China, biotechnology companies are competing to tap into the cell therapy industry. However, domestic cell therapy products still have to address the following challenges from research and development to product launch:
1. Process technology
The biological manufacturing process of cell therapy products is complicated, accompanied with high R&D and production costs and the lack of standardized quality control procedures. A typical example is autologous CAR-T, one batch of which can only be used to treat only one specific patient. It's difficult to use existing production processes to scale up production for cost reduction.
2. Capacity
In terms of domestic immune cell and stem cell therapy products, whether entering clinical trials through the filing system or through IND, it is still difficult for the cell production environment of most enterprises and institutions to meet the production plant standards with GMP qualification. Moreover, the scale-up of cell production capacity and GMP production conditions after the transition from clinical trial to commercialized production overwhelm the bearing capacity of smallest and mid-sized enterprises.
3. Talents
The explosive growth of cell therapy products has led to prominent human resource challenges in terms of qualified talents in the industry. There is a severe shortage of talents who have both the deep understanding of cellular drugs and the ability of industrial transformation, resulting in huge time and economic costs to set up a complete production line team.
4. Cost
Most of the reagents, consumables and equipment involved in the upstream production process of cell therapy products depend on imported products, suffering from high costs and the potential risk of being subjected to containment. Domestic replacement has a long way to go to step up in the industry.
【Opportunities and challenges for domestic CGT CDMO enterprises】
Faced with the above problems, CGT CDMO (Contract Development Manufacture Organization), as the upstream of CGT pharmaceutical companies, can help cell therapy companies overcome many difficulties in R&D and production, while reducing costs, to improve R&D efficiency and enhance commercialization success rate. In particular, the promotion of the domestic Marketing Authorization Holder system (MAH system), which allows the separation of drug marketing authorization from manufacturing authorization, has enabled the explosive growth of China's CGT CDMO industry in recent years.
CGT involves living cells, tissues, viral vectors and non-viral gene modification components. Its R&D technology and production process are more difficult than those of traditional biological drugs. Most of the CDMO companies in China focus on small molecular chemical drugs and macromolecular monoclonal antibody, and there are few local CGT CDMO companies. However, thanks to the increasing investment on CGT, the market demand for new therapies, and the domestic regulatory policies for the import and export of cell therapy products, traditional CDMO companies including Wuxi AppTec, Porton Pharma Solutions, Pharmaron, and OBiO Technology are rushing to enter the field of CGT CDMO. At the same time, emerging CGT CDMO companies such as Hillgene have also entered the market.
CDMO services for cell therapy products of various domestic CDMO companies in CGT industry mostly focus on immune cell therapy platform services. Currently, cell therapies represented by CAR-T are all individualized therapies. Biological manufacturing process is the bottleneck of the industry, with automation and standardization being the technical barriers.
At present, domestic companies carrying out CDMO service related to stem cell drugs are still limited to the application of traditional two-dimensional culture method for cell preparation process, and cell production capacity can only be expanded by increasing the number of culture flasks (horizontally) or by employing more layers of cell factories (vertically), which is costly in terms of manpower, time and space consumption. To make it worse, the cell quality varies greatly from batch to batch, which is difficult to meet the demand for GMP capacity expansion of cell therapy products.
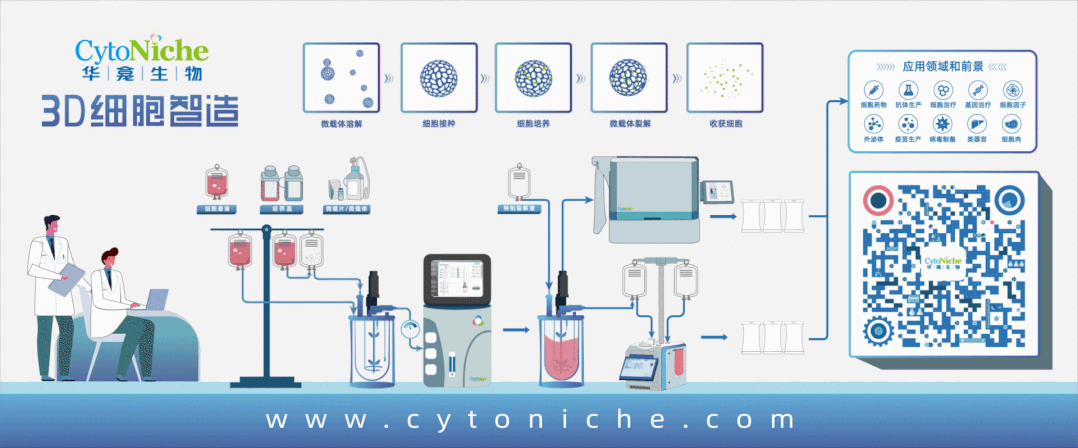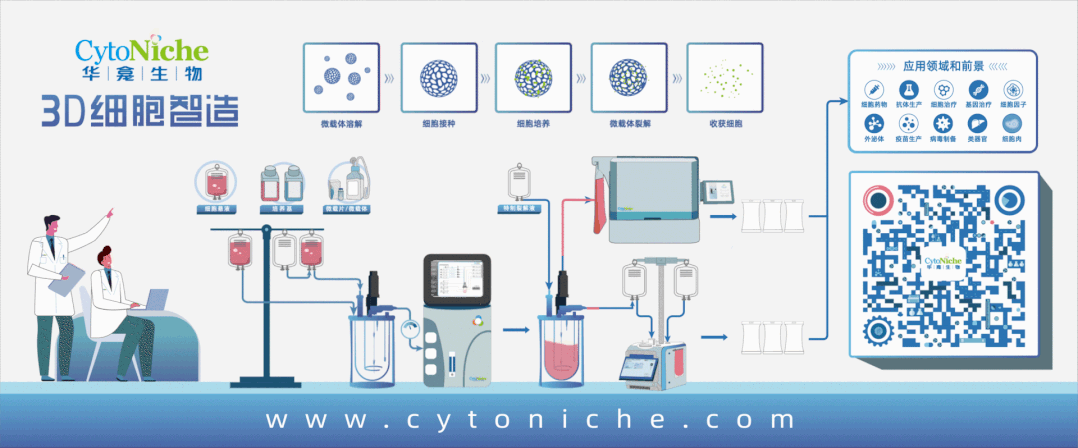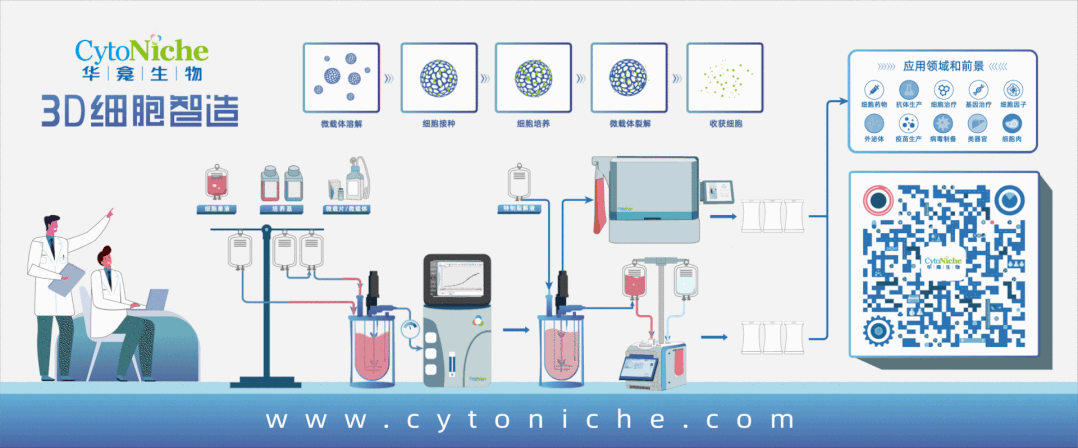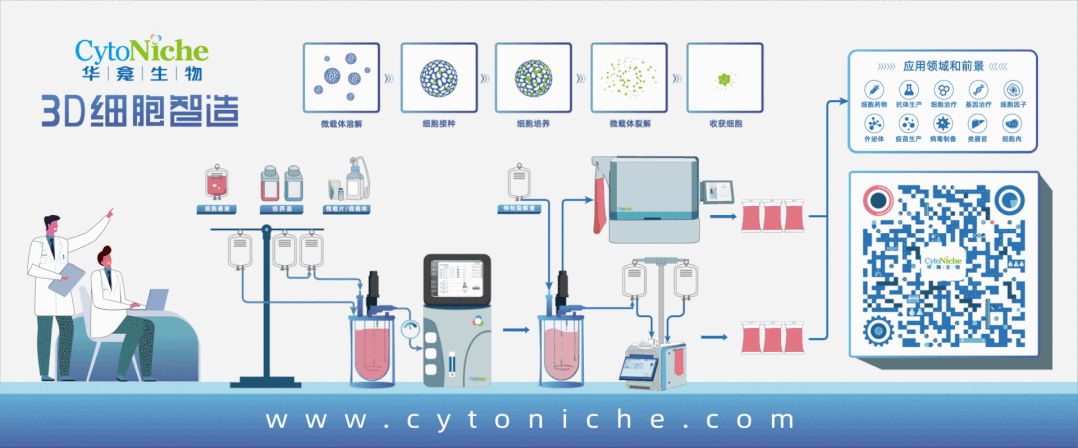
【CytoNiche 3D FloTrix® Cell Technology Platform】
Under this context, CytoNiche is also taking actions in this field, having set up the GMP-compliant cell preparation platform - 3D FloTrix® Cell Technology Platform.

Based on the unique biodegradable microcarrier technology and 3D cell preparation process of CytoNiche, a series of challenges in the clinical application of cell therapy products have been solved, including the difficulty to scale up the two-dimensional amplification culture method of adherent cells (cell factory), the huge difference of cell quality across batches, the difficulty of digestion and passage of adherent cells after the amplification and culture of adherent cells by using imported commercial microcarriers, the safety of cell end products, and the high cost of stem cell drug preparation. Meanwhile, process reproducibility and reliability are improved through automated systems (including 3D FloTrix® vivaSPIN series bioreactors and 3D FloTrix® vivaPREP cell harvesting system), and complete data traceability and continuous monitoring are allowed, enabling quality control to conform with industry regulations.
Fully automated, enclosed cell processing system is the process development trend in the field of CGT, which not only can reduce manual operations and cut production costs, but also make it easy to scale up production and control quality. It is recommended that CGT companies determine the production process at the time of IND application, so as to avoid process changing at the time of commercial production, which might end up with a series of problems involving application and validation caused by major process changes.
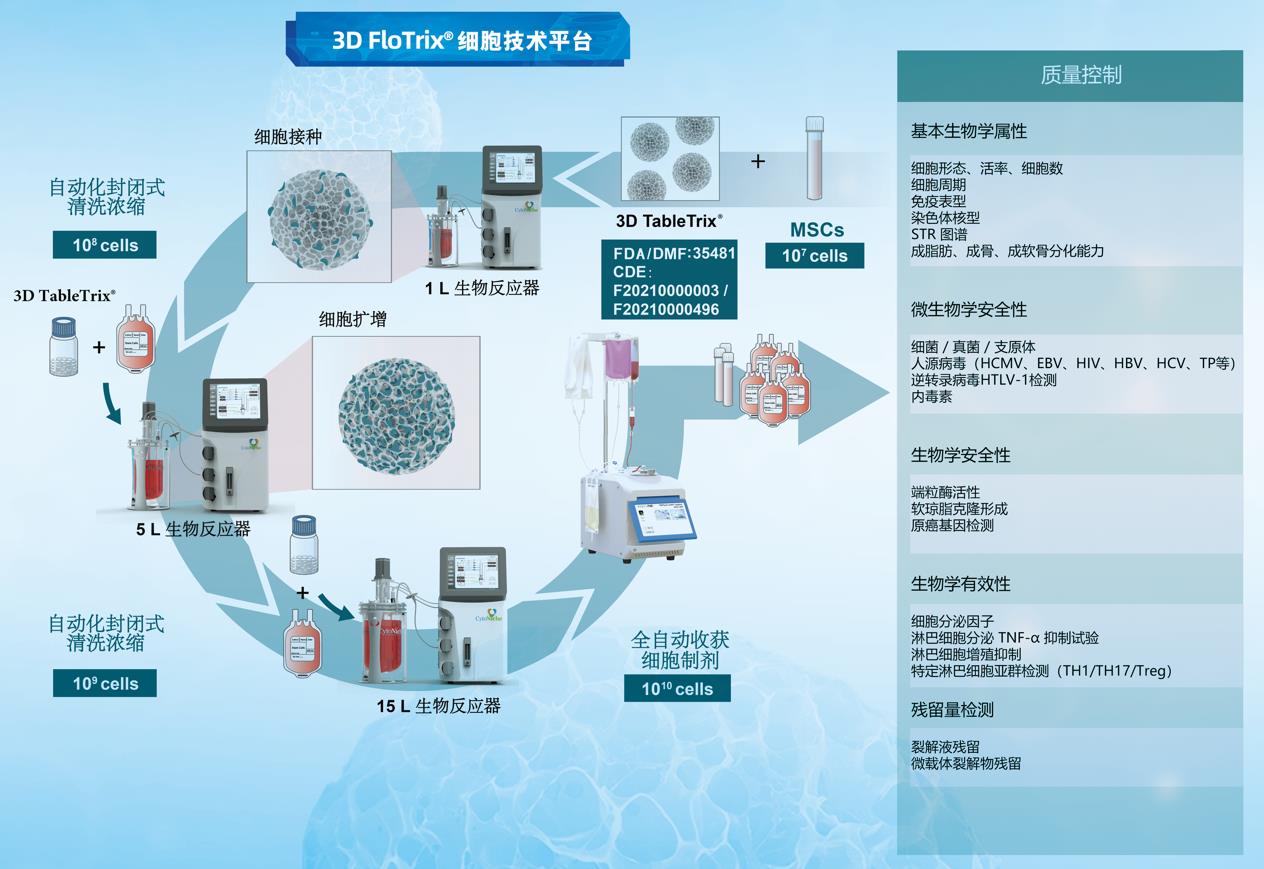
The cellular microcarrier of CytoNiche has become the world's first pharmaceutical excipient to be used in the development of cellular drugs. Such game-changing technology has been rolled out in several stem cell pharmaceutical companies and fully automated stem cell drug production lines have been established to replace the traditional manual cell production and preparation process, achieving large-scale, automated, standardized and intelligent production and preparation of stem cell drugs, as well as significantly reducing the production costs of cell therapy drugs and the cell quality differences across different batches. On this basis, we provide CDMO services that are significantly different from the existing CGT cell preparation process, including technical research, process development and GMP production services that cover mesenchymal stem cells (MSC) and exosomes from different tissue sources, to facilitate product transformation and clinical application of innovative achievements.
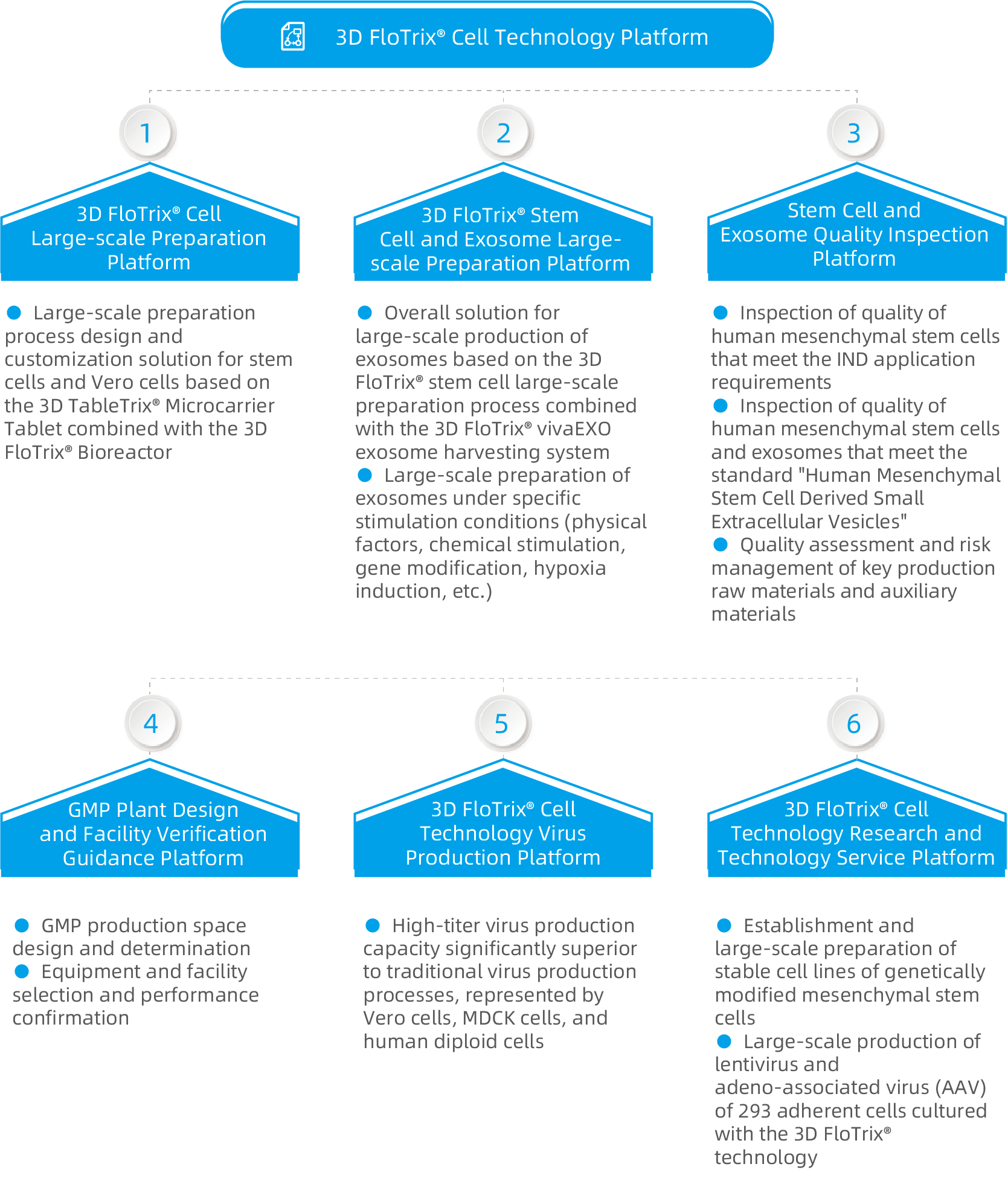
CDMO services provided by CytoNiche will be introduced in the follow-up articles.
Coming up next: GMP Production Environment of Cells
[CytoNiche]
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
