CytoNiche reaches in-depth cooperation with numerous enterprises to assist industrial upgrading and development with innovative technology
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-15
- Views:0
(Summary description)On August 8, 2022, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as "CytoNiche") held the opening ceremony of its "3D Cell Intelligent Manufacturing and Regenerative Medicine Cent
CytoNiche reaches in-depth cooperation with numerous enterprises to assist industrial upgrading and development with innovative technology
(Summary description)On August 8, 2022, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as "CytoNiche") held the opening ceremony of its "3D Cell Intelligent Manufacturing and Regenerative Medicine Cent
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-15
- Views:0
On August 8, 2022, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as "CytoNiche") held the opening ceremony of its "3D Cell Intelligent Manufacturing and Regenerative Medicine Center - 3D FloTrix® Cell CDMO Platform" at their new site after expansion.
Academician Chen Xiangmei of Chinese PLA General Hospital, Professor Du Yanan, Chief Scientist of CytoNiche, Director Xu Jian of Beijing Municipal Science and Technology Commission and Zhongguancun High-tech Industry Promotion Center, Mr. Wang Mishi, General Manager of BSTIG, and Dr. Liu Wei, CEO of CytoNiche witnessed the opening ceremony. Mr. Zhang Lin, General Manager of Technology Service Co., Ltd. of BSTIG, and Dr. Sun Yanxun, Director of CytoNiche Cell Transformation Center and Head of 3D FloTrix® Cell Technology Platform, inaugurated the "3D Cell Intelligent Manufacturing and Regenerative Medicine Center - 3D FloTrix® Cell Technology Platform" of CytoNiche.

【CytoNiche 3D Cell Intelligent Manufacturing and Regenerative Medicine Center - 3D FloTrix® Cell Technology Platform】

CytoNiche has built a fully enclosed, automated, and large-scale 3D cell preparation process development platform - 3D FloTrix® Cell Technology Platform, which covers thousands of square meters and complies with GMP standards.
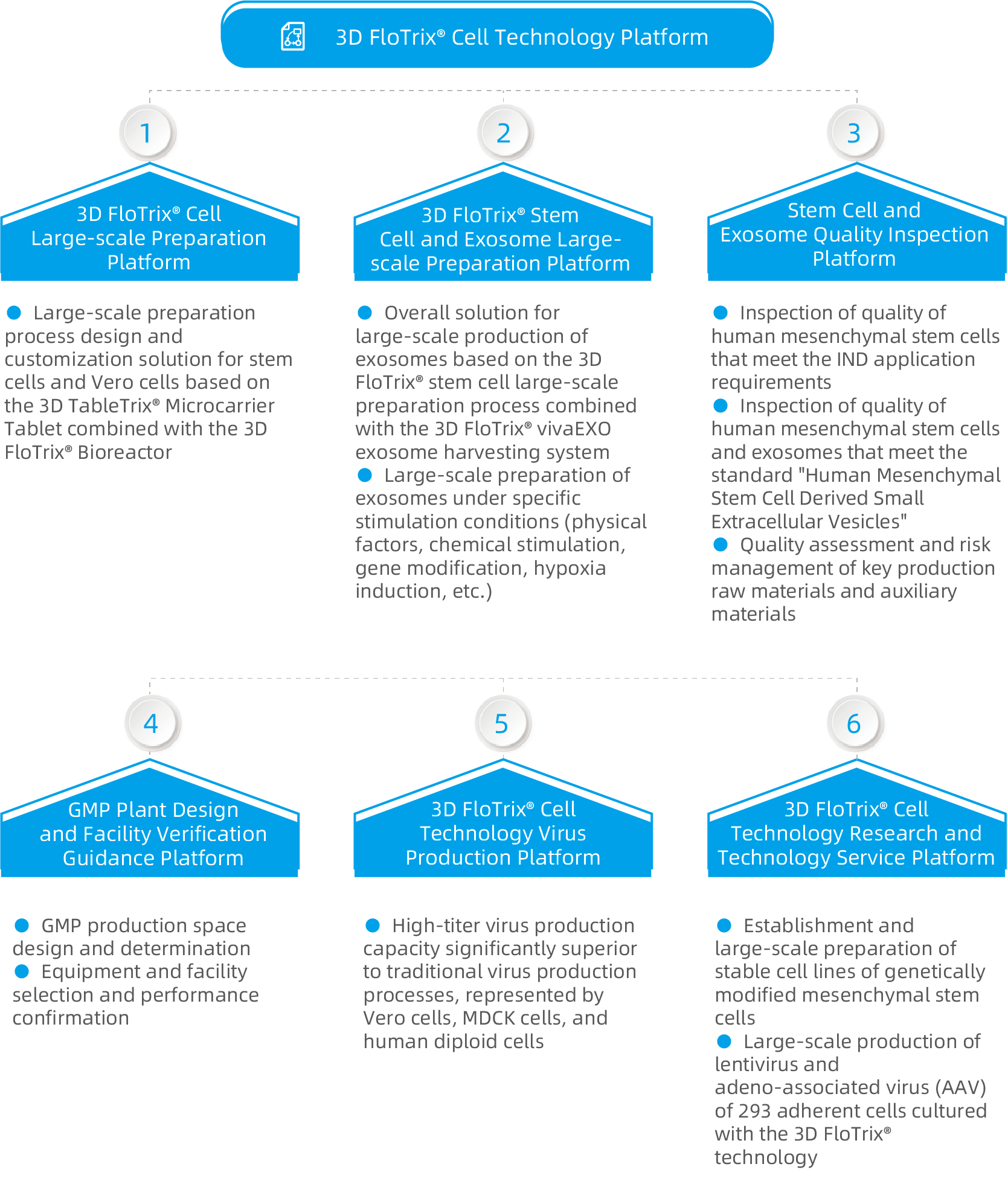
Based on the exclusive biodegradable microcarrier technology and 3D cell preparation process of CytoNiche, a series of bottleneck problems in the industrialization development of cell therapy products has been solved, assisting a number of enterprises in establishing fully enclosed and automated cellular drug production lines to realize large-scale, automated, standardized and intelligent production and preparation of cellular drugs and their derivative products. CytoNiche provides CDMO services different from the existing CGT cell preparation processes, including technical research, process development and GMP production services that cover large-scale production and preparation of mesenchymal stem cells (MSC), exosomes and viruses from different tissue sources, to facilitate product transformation and clinical application of innovative achievements.
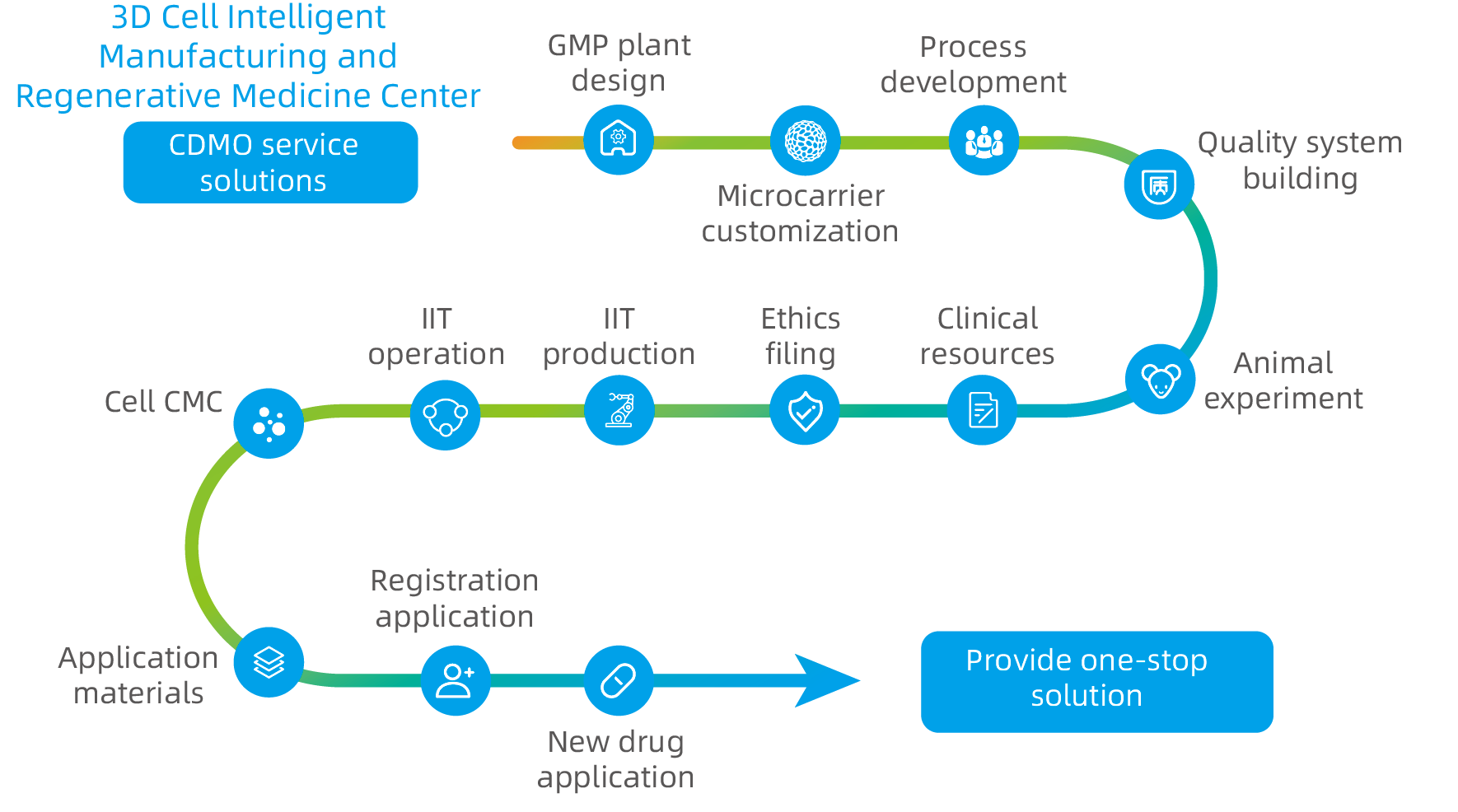
During this event, CytoNiche has reached strategic cooperation with Sinovac Biotech Ltd. (Sinovac), Wuhan Canvest Biotechnology Co., Ltd. (Canvest), and Beijing SAFE Pharmaceutical Technology Co., Ltd. (SAFE Pharmaceutical), three leading companies in the biopharmaceutical field.
Based on the cooperation at this stage, CytoNiche will work with these companies to promote technological innovation and upgrading, as well as product application and transformation, through 3D cell "intelligent" manufacturing platform and customized one-stop solution. In addition, CytoNiche will cooperate with multiple forces in the upstream, mid-stream and downstream of the industrial chain to further address industry challenges and accelerate the clinical transformation of drugs.
Join hands with Sinovac to develop the cooperation platform for research and development of 3D microcarrier vaccine
Sinovac values "Supply Vaccines to Eliminate Human Diseases" as its mission and is committed to providing services for disease prevention and control through the research, development, production and sales of vaccines and related products.
CytoNiche and Sinovac have reached this strategic cooperation to jointly develop an innovative vaccine industry line by conducting research on microcarrier process changes based on the existing vaccine industry line. The two parties will employ their respective resources to jointly establish industrial alliances to promote the upgrading of the upstream vaccine industry.

Prof. Du Yanan, Chief Scientist of CytoNiche, Dr. Liu Wei Liu, CEO of CytoNiche, and Mr. Keyimu, Director of Strategic Cooperation Center of CytoNiche witnessed the onset of this cooperation. Mr. Han Xing, Production Director of Sinovac, and Dr. Yan Xiaojun, CTO of CytoNiche inaugurated the cooperation between the two parties.
Join hands with Canvest to develop the quality and safety system for cell innovation products
Canvest is a high-tech enterprise integrating biotechnology services, research and development. The company is dedicated to providing all kinds of cells (including recombinant cells, stem cells, and immune cells) and technical services including quality inspection of raw materials and virus removal process validation, to production enterprises, and research and development institutions of biological drugs/products.
CytoNiche and Canvest signed the cooperation agreement. The two parties will jointly establish a quality evaluation system for large-scale 3D cell production of cell preparations based on Canvest's expertise in cell quality review and inspection, aiming to lay a solid foundation for the subsequent pharmaceutical research and development application, and industrial production of cell preparations based on large-scale 3D cell production and intelligent manufacturing.

Join hands with SAFE Pharmaceutical to serve the demand of the whole industrial chain for cell innovation products
SAFE Pharmaceutical is committed to becoming a world-class one-stop CRO service platform for pharmaceutical innovation drugs. The core business of CGT innovation drug research and development covers the whole field of pharmacological and pharmacodynamics effects, animal model, drug metabolism analysis, non-clinical safety evaluation, and central laboratory, providing comprehensive solutions for innovative pharmaceutical enterprises to enable rapid business growth.
CytoNiche joins hands with SAFE Pharmaceutical to develop an integrated, one-stop whole industry chain service system for cellular drugs that conforms to international standards, and facilitate global cellular drug research and development by adopting high-standard and high-quality technical system and service capacity.

Prof. Du Yanan, Chief Scientist of CytoNiche, Dr. Yan Xiaojun, CTO of CytoNiche, Dr. Sun Yanxun, Director of CytoNiche Cell Transformation Center and Head of 3D FloTrix® Cell Technology Platform, and Mr. Keyimu, Director of Strategic Cooperation Center of CytoNiche witnessed the onset of this cooperation. Mr. Liu Yang, President of SAFE Pharmaceutical, and Dr. Liu Wei, CEO of CytoNiche inaugurated the cooperation between the two parties.
During this event, CytoNiche, Beijing Baylx Biotech Co., Ltd. (Baylx Biotech) and Jiangsu Topcel Kangheng Pharmaceutical Co., Ltd. (Topcel Kangheng) established the "3D Cell Intelligent Manufacturing Joint Laboratory" by employing the advantages of each party.
Based on the cooperation at this stage, CytoNiche will work with these companies to promote technological innovation and upgrading, as well as product application and transformation, through 3D cell "intelligent" manufacturing platform and customized one-stop solution. In addition, the parties will jointly create innovative technology products originating from China and leading in the world.
Work with Baylx Biotech to explore novel cell therapy solutions
Baylx Biotech is a high-tech enterprise focusing on the research of stem cell and regenerative medicine as well as new drug development and clinical transformation of stem cells. The company is committed to the new drug development and clinical research of stem cells. At present, two types of "Human Umbilical Cord Mesenchymal Stem Cell Injections" have been approved for the investigational drug application by the National Medical Products Administration to start clinical trials.
By integrating respective superior resources, CytoNiche and Baylx Biotech have developed and set up a research and development platform centering on microcarrier 3D cell application technology, dedicating to breaking through technical bottlenecks and creating innovation products to provide novel cell therapy solutions that address previously unsatisfied clinical needs faced by mankind.

Prof. Du Yanan, Chief Scientist of CytoNiche, Dr. Liu Yongjun, Chairman of Baylx Biotech, Dr. Liu Wei, CEO of CytoNiche, Dr. Yan Xiaojun, CTO of CytoNiche, and Mr. Keyimu, Director of Strategic Cooperation Center of CytoNiche witnessed the onset of this cooperation. Dr. Liu Guangyang, Technical Director of Baylx Biotech, and Dr. Sun Yanxun, Director of CytoNiche Cell Transformation Center and Head of 3D FloTrix® Cell Technology Platform inaugurated the cooperation between the two parties.
Work with Topcel Kangheng to develop the production platform for 3D large-scale cell amplification
Topcel Kangheng specializes in the development and clinical transformation of stem cell drugs. It has conducted clinical studies for several indications. At present, the company has obtained one clinical implied license for the registration of stem cell drugs, and their application for another one is under review. Topcel Kangheng is dedicated to developing "high-quality, low-cost, large-scale and reproducible" commercial production process of stem cells. At present, a number of enclosed, large-scale stem cell preparation process studies have been carried out.
CytoNiche joins hands with Topcel Kangheng to cooperate in 3D microtissue engineering technology, large-scale culture and amplification process of cells, and new drug research and development of cellular microtissue, aiming to build a production platform of 3D large-scale cell amplification based on degradable microcarriers.

Prof. Du Yanan, Chief Scientist of CytoNiche, Dr. Yan Xiaojun, CTO of CytoNiche, Dr. Sun Yanxun, Director of CytoNiche Cell Transformation Center and Head of 3D FloTrix® Cell Technology Platform, and Mr. Keyimu, Director of Strategic Cooperation Center of CytoNiche witnessed the onset of this cooperation. Dr. Lian Yunfei, General Manager of Topcel Kangheng, and Dr. Liu Wei, CEO of CytoNiche inaugurated the cooperation between the two parties.
【Cooperation for mutual benefits】
Being able to reach various strategic cooperation and set up a joint laboratory,is the fruit of joint efforts by both parties.
On the basis of complementary advantages and resource sharing,the two parties are binding more closely together,to jointly promote the industry development and serve for human life and health.
About Sinovac

Sinovac Biotech Ltd. (Sinovac) is a leading biotech company in China. Sinovac values "Supply Vaccines to Eliminate Human Diseases" as its mission and is committed to providing services for global disease prevention and control through the research, development, production and sales of vaccines and related products.
The vaccines developed by Sinovac mainly include vaccines against emerging and outbreak of infectious diseases such as SARS, avian influenza (H5N1), 2009 influenza A (H1N1), EV71 hand, foot and mouth disease, and COVID-19, as well as vaccines against common infectious diseases such as viral hepatitis, influenza, pneumonia, poliomyelitis, chickenpox and mumps. Among these vaccines, CoronaVac®, the inactivated vaccine for COVID-19, has achieved a global supply of over 2.7 billion doses.
About Canvest

Established in 2011, Wuhan Canvest Biotechnology Co., Ltd. (Canvest) is a high-tech enterprise integrating biotechnology services, research and development. The company is dedicated to providing all kinds of cells (including recombinant cells, stem cells, and immune cells) and technical services including quality inspection of raw materials and virus removal process validation, to production enterprises, and research and development institutions of biological drugs/products.
About SAFE Pharmaceutical

SAFE Pharmaceutical is committed to becoming a world-leading one-stop medical CRO service company by providing first-class medical CRO evaluation services. The company also helps enterprises in overall innovative pharmaceutical research and development planning through services involving technology development, financing, and transformation of innovative pharmaceutical products, accelerating the research and development process of innovation drugs to enable faster growth of enterprises.
The company is able to provide professional, one-stop medical research and development services for pharmaceutical research and development enterprises through its technical departments such as pharmaceutical research and development consultation, NMPA/FDA registration and application, compound screening, druggability evaluation, animal models, pharmacology and efficacy, drug metabolism analysis, non-clinical safety evaluation, pharmaceutical preparations, biological sample analysis, clinical phase I and pharmacovigilance.
The company has been awarded the honorary titles of National High-tech Enterprise, Beijing "Little Giant" Enterprise that is specialized and sophisticated in producing new and unique products, Beijing Enterprise Science and Technology R&D Organization, Beijing Small and Medium-sized Sci-tech Enterprise, and Zhongguancun High-tech Enterprise. The company also owns two post-doctoral research stations.
SAFE Pharmaceutical has Jingnan Gu'an Drug GLP Center, Pharmacology and Efficacy Center, Shenzhen DrugEvaluation Center, Chengdu Animal Models Center, Compound Screening Center, Beijing Clinical Evaluation Center, Central Laboratory, Shenyang Biological Sample Analysis Center, four self-established GCP Clinical Trial Bases for Grade-A tertiary hospital, and SAFE Pharmaceutical US office that is responsible for FDA registration and application.
About Baylx Biotech

Baylx Biotech is a high-tech enterprise focusing on the research of stem cell and regenerative medicine as well as new drug development and clinical transformation of stem cells. The company is committed to the new drug development and clinical research of stem cells. At present, two types of "Human Umbilical Cord Mesenchymal Stem Cell Injections" have been approved for the investigational drug application by the National Medical Products Administration to start clinical trials. The company has independently developed more than 20 genetically modified stem cell products for fighting solid tumors, forming a combination therapy system for solid tumors, and has technical advantages as well as an independent intellectual property rights system in this field.
About Topcel Kangheng

In order to promote the development of cell industry in Jiangsu Province, Jiangsu Commission of Health, Jiangsu Medical Products Administration, Jiangsu Development and Reform Commission, and Jiangsu Science and Technology Department jointly established the "Jiangsu Regional Cell Preparation Center" (operated by Jiangsu Topcel Kangheng Pharmaceutical Co., Ltd.). The main business of the center includes: carrying out standardized research on cell collection, preparation and quality control; drafting standards and specifications for cell products; developing a transformation platform for cell therapy applications. At present, the center is beginning to take shape. Phase I of the laboratory covers an area of 3000 square meters, and a quality management system and cell preparation workshop complying with international ISO9001 standards and EU GMP standards have been set up. In early 2021, the center obtained China’s first "Drug Manufacturing Certificate" for stem cell drugs and NK cells drugs.
Jiangsu Topcel Kangheng Pharmaceutical Co., Ltd. specializes in the development and clinical transformation of stem cell drugs. It has conducted clinical studies for several indications. At present, the company has obtained one clinical implied license for the registration of stem cell drugs, and the application for another one is under review. Products for other indications are also in the fast track of development and application. Topcel Kangheng is dedicated to developing "high-quality, low-cost, large-scale and reproducible" commercial production process of stem cells. At present, a number of enclosed, large-scale stem cell preparation process studies have been carried out.
About CytoNiche

Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. Their core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D "intelligent manufacturing" platform for cells, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
The core product of CytoNiche, 3D TableTrix® Microcarrier (microcarrier), is an independent innovation and the first pharmaceutical excipient microcarrier that can be used for cell drug development. CytoNiche has passed the inspection of the National Institutes for Food and Drug Control and other relevant authorities, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). In addition, the product has obtained the DMF qualification for pharmaceutical excipient from FDA (DMF: 35481).
The products and services of CytoNiche can be widely applied in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines and protein products. They also have broad application prospect in regenerative medicine, organoid and food technology (cell-cultured meat, etc.).
The company has 5000 square meters of research and development, and transformation platform, including 4000 square meters of GMP production platform, more than 1000 square meters of CDMO service platform focusing on 3D cell intelligent manufacturing and micro-tissue regenerative medicine treatment products; a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
