Major Breakthrough: 3D FloTrix® Serum-Free Culture Medium for Mesenchymal Stem Cells Officially Registered with 【DMF】
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-07-06
- Views:189
(Summary description)DMF Registration Number: 038476, Facilitating Clinical Submissions for Pharmaceutical Companies!
Major Breakthrough: 3D FloTrix® Serum-Free Culture Medium for Mesenchymal Stem Cells Officially Registered with 【DMF】
(Summary description)DMF Registration Number: 038476, Facilitating Clinical Submissions for Pharmaceutical Companies!
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-07-06
- Views:189
CytoNiche Bio's 3D FloTrix® Serum-Free Culture Medium for Mesenchymal Stem Cells has successfully obtained a Drug Master File (DMF) registration with the U.S. Food and Drug Administration (FDA), with DMF registration number: 038476.

Click the image to view product details.
▣ Supports primary isolation and passaging cultures
▣ Suitable for both 2D and 3D cell culture models
▣ Applicable to various mesenchymal stem cell cultures
▣ One choice to meet different process requirements

[About DMF]
The U.S. DMF (Drug Master File) guidance is a submitted archive of pending materials to the FDA (the authoritative regulatory agency for global pharmaceuticals). It was implemented in 1989 and has been in use ever since.
DMF contains confidential information about facilities, operations, packaging, storage processes, and details of substance usage during production and operation. It can be used to support Investigational New Drug Applications (IND), New Drug Applications (NDA), Abbreviated New Drug Applications (ANDA), another DMF, or export applications. The significance and importance of DMF filing lie in providing pharmaceutical companies a reliable platform to demonstrate product quality and safety to regulatory authorities.
It also provides regulatory agencies with a means to supervise and review drugs. Obtaining DMF registration is another significant qualification achieved for the 3D FloTrix® Serum-Free Culture Medium for Mesenchymal Stem Cells, demonstrating that CytoNiche Bio's production process and quality control system have met international standards and regulatory requirements.
[CytoNiche's Support for Submissions]
For numerous pharmaceutical clients, utilizing CytoNiche Bio's DMF registration number instead of providing specific information about raw materials and excipients during the submission process can:
▣ Reduce the time required for data preparation, review, and evaluation
▣ Greatly save on approval costs and enhance approval efficiency
▣ Shorten the drug registration cycle, accelerating clinical/market submissions
[CytoNiche's Support Services]
▷ If you are using the 3D FloTrix® Serum-Free Culture Medium for Mesenchymal Stem Cells for related research projects and need to submit applications to the FDA such as Investigational New Drug Applications (IND) or New Drug Applications (NDA), you can contact relevant sales representatives to apply. CytoNiche Bio will provide you with an authorization letter that permits the FDA to directly review the DMF technical content involved in the drug application review process, assisting you in expediting the FDA review process.
▷ If you are interested in CytoNiche Bio's 3D FloTrix® Serum-Free Culture Medium for Mesenchymal Stem Cells, you can also scan the code to apply for a trial.

【CytoNiche】
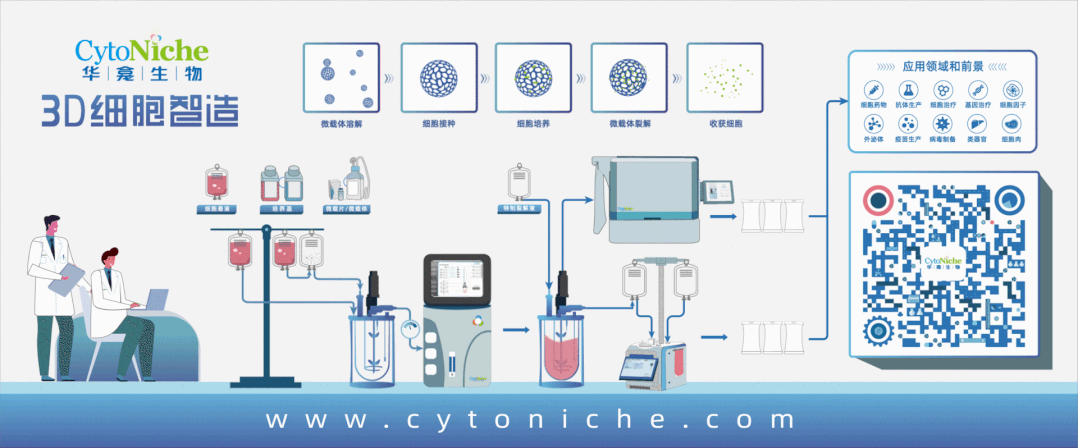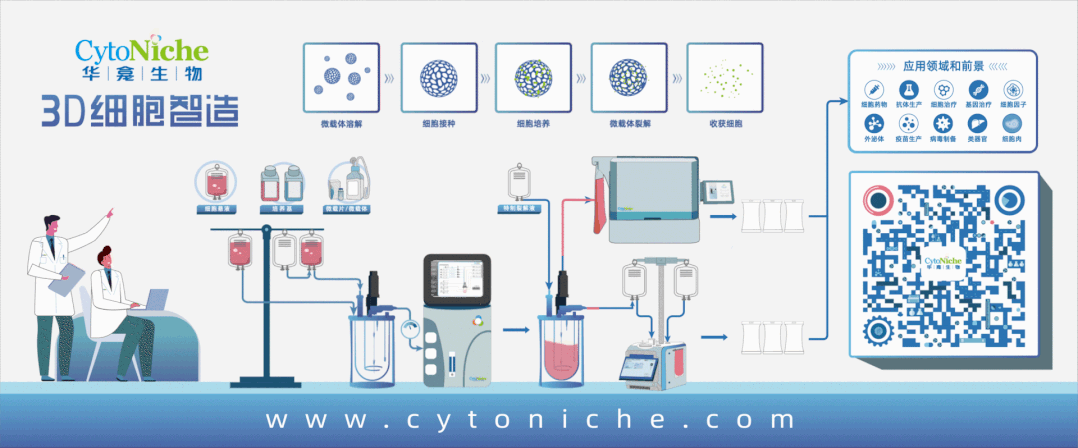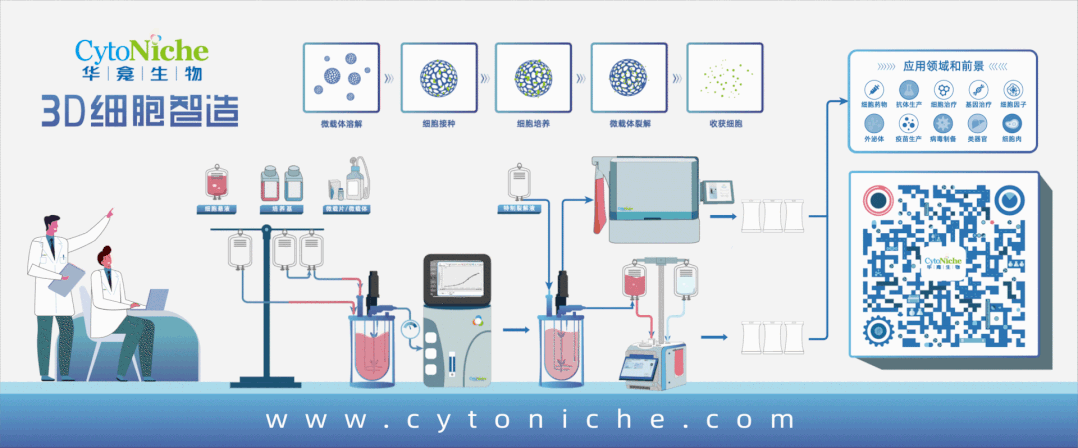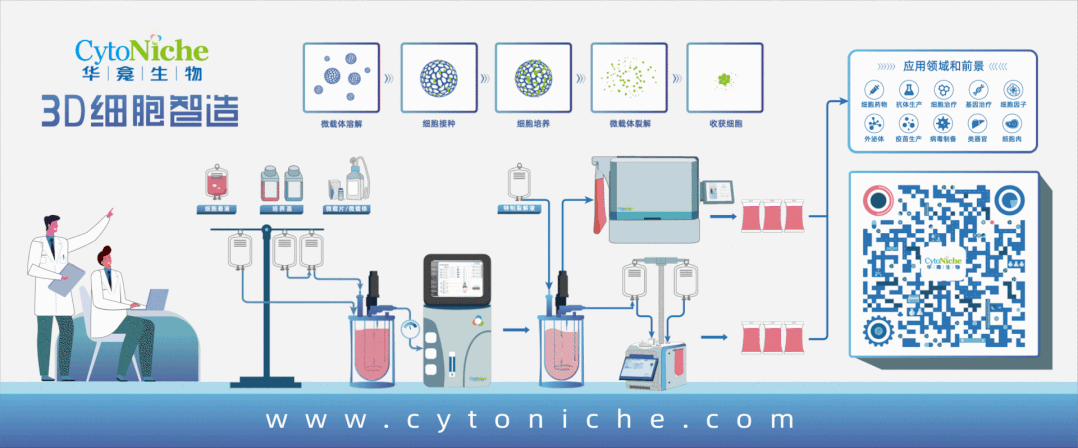
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
