Application of Novel 3D Porous Biodegradable Microcarrier in the Vaccine Field
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-07-11
- Views:21
(Summary description)The utilization of 3D TableTrix® Microcarrier V01 in conjunction with 3D FloTrix® Automated Bioreactor presents a more efficient large-scale suspension culture method for Vero cells, offering a new ch
Application of Novel 3D Porous Biodegradable Microcarrier in the Vaccine Field
(Summary description)The utilization of 3D TableTrix® Microcarrier V01 in conjunction with 3D FloTrix® Automated Bioreactor presents a more efficient large-scale suspension culture method for Vero cells, offering a new ch
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-07-11
- Views:21
[Background]
In traditional cultivation methods, adherent cells are typically cultured using cell culture flasks, roller bottles, or cell factories, which have limited scale, difficulty in process control, and challenges in upscaling while maintaining quality control. The combination of microcarriers with bioreactors for suspension culture of adherent cells provides a compelling solution. CytoNiche Biotech introduces the first 3D porous biodegradable microcarrier, 3D TableTrix® Microcarrier V01, suitable for adherent cell expansion, passaging, and virus production.

3D TableTrix® Microcarrier V01
Compared to traditional microcarriers, there are significant advantages in cell expansion and virus production. When used for large-scale cultivation of Vero cells, cell densities can reach above 1 × 10^7 cells/mL. Cells can be stably amplified step by step within the bioreactor, ultimately achieving high-titer production of both secreted and intracellular viruses.
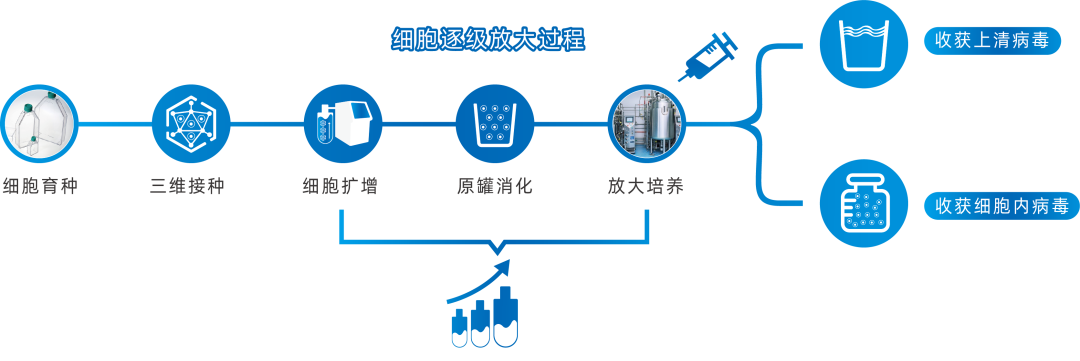
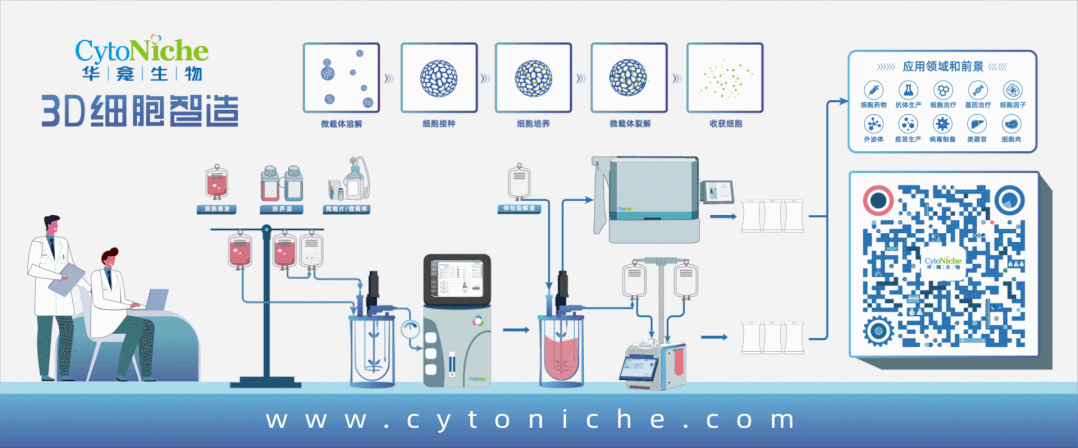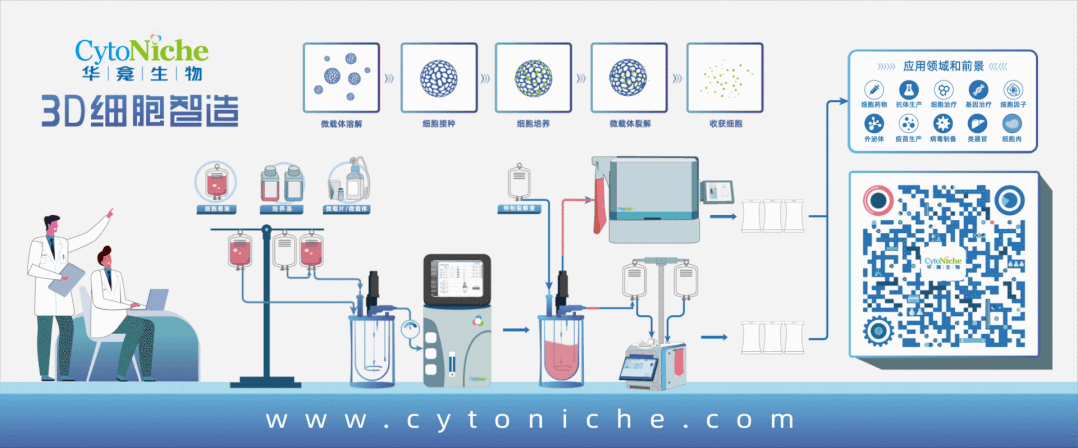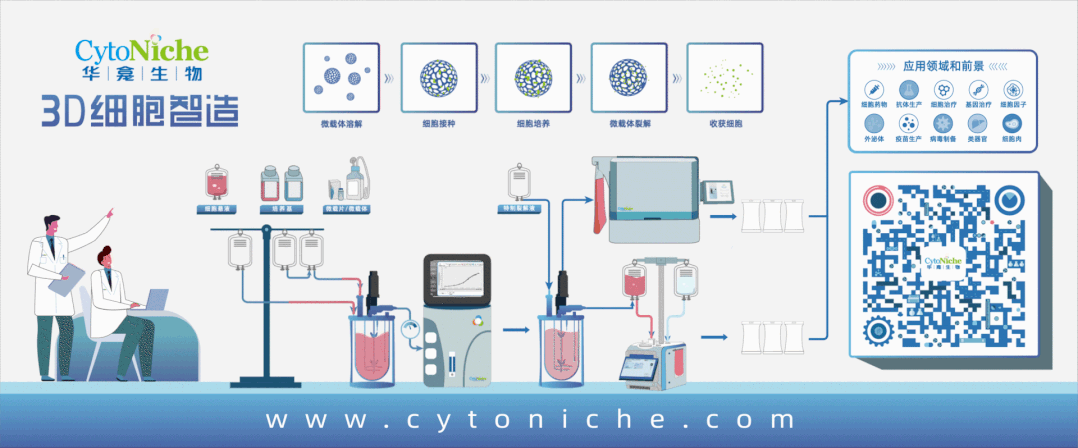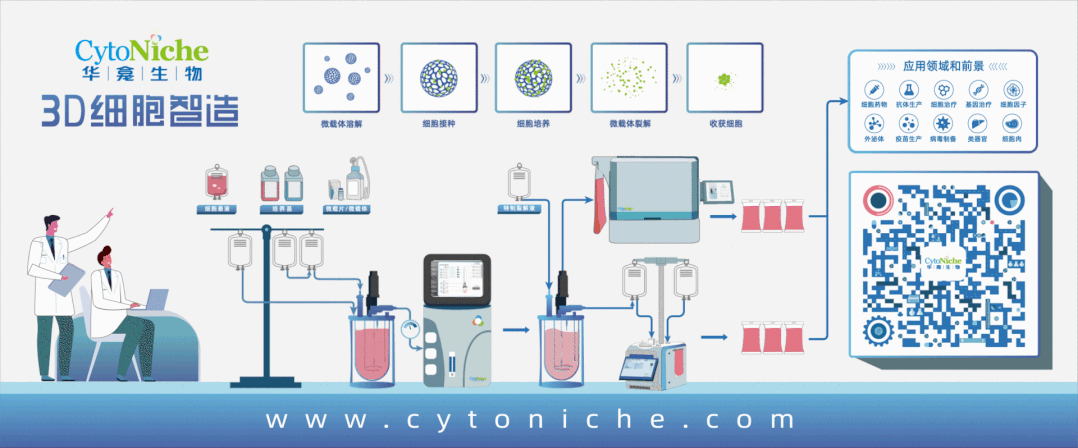
Figure 1: 3D FloTrix® Cell Large-Scale Cultivation Technology for Virus Production
[Features]
1.Honeycomb-like porous structure with pore sizes ranging from 30 to 50 μm, porosity > 90%.
2.Extremely high surface area (>9000 cm2/g), equivalent to 2-3 times the surface area of traditional microcarriers, offering more effective growth space at the same feeding density to accommodate more adherent cells.
3.Fully biodegradable microcarrier with a validated residual degradation detection method (reagent kit) from China National Institutes for Food and Drug Control (NIFDC), enabling non-damaging cell harvesting, serving as an alternative to cell factories for cell cultivation and seed bank establishment.
4.Possesses biomimetic biomechanical properties, effectively buffering fluid shear forces and collision between microcarriers in stirred bioreactors, better safeguarding cell viability and thereby enhancing virus production.
5.Simple upscaling process, cells can be scaled up from one vessel to another, closed-system process effectively mitigates contamination risk.
6.Supports steam sterilization, suitable for large-scale production in stainless steel reactors.
Part 1: Establishment of Vero Cell Large-Scale Culture Process Using Microcarriers
● Cell propagation: Using 10-layer cell factories, cells are cultured for 72 hours before inoculation into the bioreactor.
● Culture medium: Inoculation medium contains 10% NBS in SFM medium, and exchange medium contains 1% NBS in SFM medium.
● Microcarrier: 3D TableTrix® Microcarrier V01, density: 2 g/L.
● Cell density: Recommended cell inoculation density is 250,000 - 400,000 cells/mL under microcarrier density of 2 g/L.
● Inoculation conditions: Cycling between 40 rpm for 5 minutes and 0 rpm for 25 minutes for 8-16 hours, followed by constant speed at 45 rpm.
● Cell cultivation: Daily sampling for cell counting and glucose content measurement. Exchange medium containing 80% of total culture volume is used when glucose content drops below 1 g/L.
● Upscaling ratio: 1:5.
● In situ passaging:When cell proliferation reaches 8-10 fold, in situ passaging can be conducted. Microcarriers are counted before passaging using trypsin digestion. Following counting, microcarriers are sedimented, washed with PBS 3 times, treated with cell digestion enzyme for approximately 30 minutes to detach cells, and fully supplemented culture medium is used to terminate digestion. Appropriate volume of cell-microcarrier suspension is transferred to the next-level bioreactor based on required cell amount for the next passage.
● Third-stage proliferation results:
◉ Vero cells can achieve three stages of amplification, and cell proliferation is stable.
◉ The highest cell density reached during 8 days of growth is 1.37 × 108 cells/mL (Figure 2).
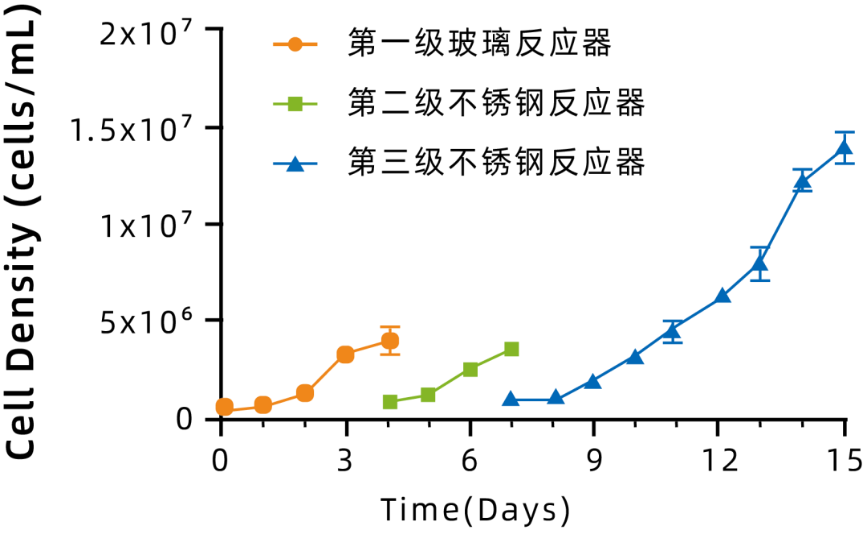
Figure 2: Vero Cell Large-Scale Amplification Proliferation Curve
◉ First-stage transfer vessel recovery rate: 93.8%;
◉ Second-stage transfer vessel recovery rate: 100%, and cells maintain high activity (Figure 3).
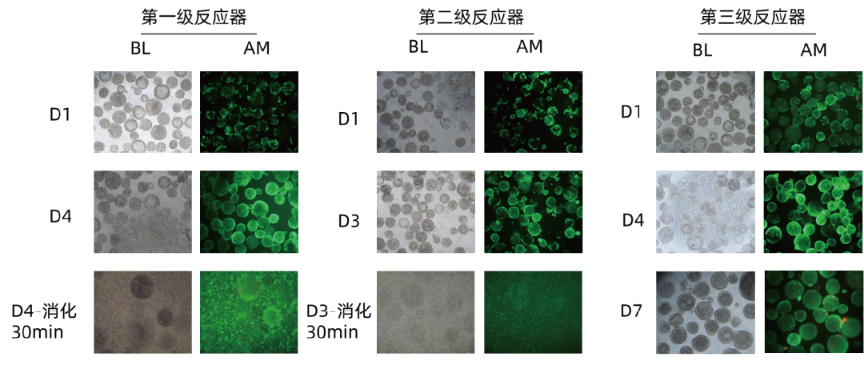
Figure 3: Vero Cell Large-Scale Amplification Proliferation and Digestion Fluorescence Image
Part 2: Optimization of 3D Microcarrier for Large-Scale Cultivation of Vero Cells
In order to further reduce economic costs and operational complexity, optimizations were made to the original inoculation process by adjusting the composition of the inoculation and replacement culture media. The serum concentration in the inoculation medium was lowered to 3%, and the replacement culture medium was formulated without serum. Additionally, the cultivation ratio was further increased to 1:8.
As serum was omitted during media replacement, the number of washing steps during passaging was reduced from three times to one time, resulting in effective savings in reagent consumables and operation time. Following the process optimization, the amplification outcomes were as follows: the absence of serum did not compromise cell proliferation efficiency, and a cell density of 5.42 × 10^6 cells/mL was achieved after 5 days of growth (Figure 4). During passaging, a single PBS wash did not affect digestion efficiency, with a transfer vessel recovery rate of 93.3% and cells maintaining relatively high activity.
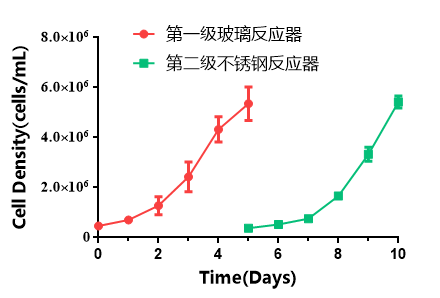
Figure 4: Proliferation Curve after Process Optimization
Part 3: Application of 3D Microcarrier for Porcine Epidemic Diarrhea Virus (PEDV) Production
After cultivating Vero cells on the 3D TableTrix® Microcarrier V01 for 72 hours (Day 3), the cell density increases to around 2.5 × 10^6 cells/mL. Subsequently, the cells are inoculated with Porcine Epidemic Diarrhea Virus (PEDV). Following the inoculation, the supernatant is collected daily to measure the peak virus titer, and a comparison is made with virus production using traditional two-dimensional cell culture methods.
Results for PEDV production: After the virus is inoculated, the cells on the 3D TableTrix® Microcarrier gradually undergo pathological changes and detach. By 160 hours post-inoculation, the cells on the microcarrier are almost entirely detached (as shown in Figure 5).
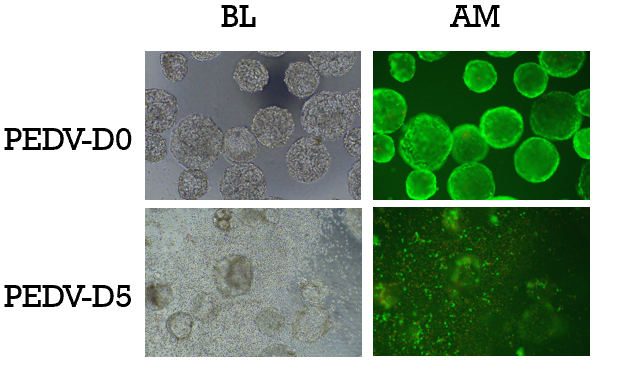
Figure 5. Cellular State Before and After PEDV Inoculation
The highest virus titer reached 108.57 TCID50/mL, which is more than 100 times higher than the highest titer achieved using the 2D planar process (as shown in Figure 6).
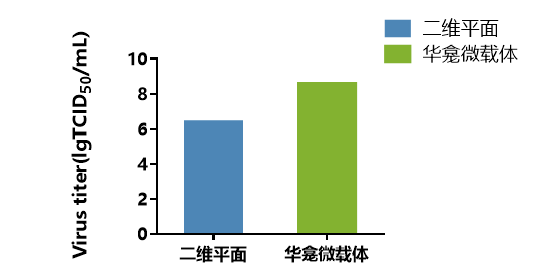
Figure 6. Comparison of PEDV Titers between Two Cultivation Methods
Part 3. Advantages of Microcarrier Utilization in Large-Scale Vero Cell Cultivation
1.Rapid Cell Proliferation: A 10-fold increase in cell proliferation can be achieved within 5 days.
2.High Cell Recovery Rate: Over 90% cell recovery ensures a substantial amplification ratio.
3.Convenient Scale-Up: Transfer operations are simple, requiring only 1-2 personnel, leading to reduced labor costs.
4.Reduced Serum Usage: Minimal serum requirement effectively lowers costs.
5.Enhanced Virus Production: Significant enhancement in virus titer, resulting in improved virus production efficiency.
Based on the large-scale Vero cell cultivation process utilizing CytoNiche's microcarrier technology, under the conditions of microcarrier density of 2g/L and cell seeding density of 300,000 cells/ml, a 10-fold cell proliferation can be achieved within 5 days using serum-free culture medium. The process includes cell passaging through vessel-to-vessel transfer, achieving cell recovery rates consistently above 90%.
In the application of this process to Vero cell cultivation for porcine epidemic diarrhea virus (PEDV) production, the virus titer achieved is significantly higher than that of 2D planar cell culture. This optimized process employs minimal microcarrier usage and offers operational simplicity, while also allowing for microcarrier degradation and efficient cell harvest, thus catering to various vaccine production process requirements.
[Summary]
The performance of 3D TableTrix® Microcarrier V01 in Vero cell cultivation is notably advantageous. Compared to existing cultivation methods, the use of relatively low-density V01 microcarriers facilitates large-scale cell expansion. Additionally, these microcarriers can be degraded, enabling non-destructive cell harvesting, while the process itself remains straightforward and scalable.
Leveraging the distinctive advantages of 3D TableTrix® Microcarrier V01 in conjunction with the 3D FloTrix® automated bioreactor significantly reduces costs associated with labor, time, and instrument consumables, while simultaneously meeting the vaccine industry's requirements for cell quantity and quality. This innovation provides vaccine enterprises with new options and a fresh impetus.
[Research Application Materials]
Core Product: 3D TableTrix® Microcarrier from CytoNiche
|
|
 |
①Biomimetic: Composed of tens of thousands of elastic three-dimensional porous microcarriers, with a porosity >90%, controllable particle size in the range of 50-500μm, and uniformity ≤100μm, creating a truly biomimetic 3D culture environment.
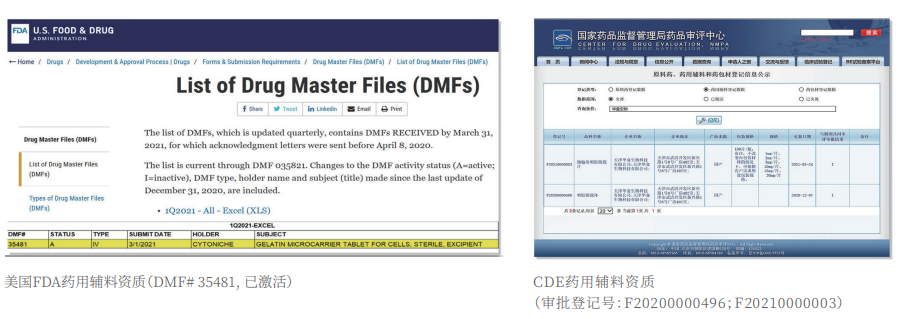
②Certified: The product has obtained two qualifications as a drug excipient from the China Drug Evaluation (CDE), with registration numbers [F20200000496; F20210000003], and one qualification as an FDA Drug Master File (DMF) excipient, with registration number [DMF:35481].
3D FloTrix® Digest Solution
|
|
 |
3D FloTrix® Digest Solution
③ Easy Harvest: Specific degradation technology to digest microcarriers, enabling more efficient and gentle cell harvest compared to traditional methods.
Authoritative Testing Reports
④ Safer: Equipped with quality evaluation reports from authoritative institutions, including residual digestion detection, cell toxicity, pyrogen reaction, genetic toxicity, in vivo immunotoxicology, as well as evaluations for hemolysis, subcutaneous injection local irritation, systemic allergenicity, and peritoneal injection toxicity.
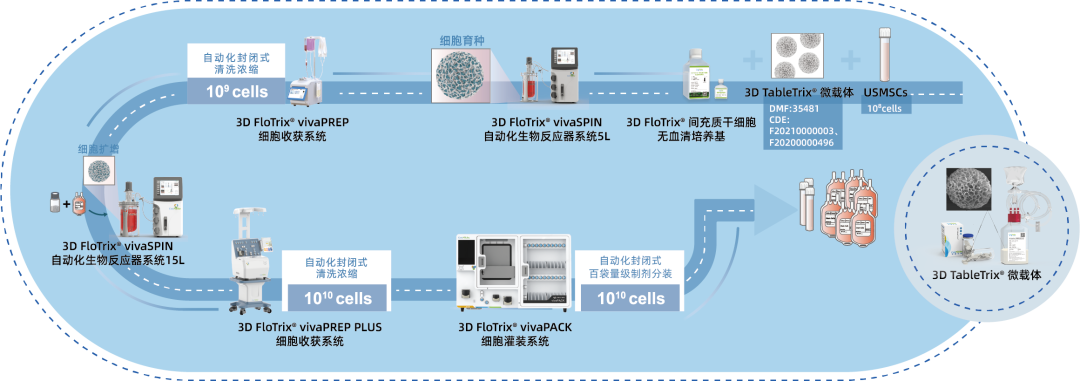
⑤ Scalable: By utilizing 3D culture techniques and combiningCytoNiche Bio's 3D Cell Intelligent Manufacturing Platform, large-scale cell culture can be achieved automatically in a closed system, allowing for the harvest of billions of cells.

If you have any questions or needs about this product, you can scan the code or call the hotline: 400-012-6688
We will ask technical experts to provide you with answers and help.
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP





 京公网安备 11010802037749号
京公网安备 11010802037749号
