"Bioactive Materials" Major Research Achievement Published: Dual-functional in-situ self-assembling organoids based on 3D microcarriers for bone-cartilage regeneration
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-07-04
- Views:189
(Summary description)Using 3D TableTrix® microcarriers as a foundation, personalized gelatin microcarriers were designed in vitro, which self-assembled in vivo to form bone-cartilage organoids. These organoids were employ
"Bioactive Materials" Major Research Achievement Published: Dual-functional in-situ self-assembling organoids based on 3D microcarriers for bone-cartilage regeneration
(Summary description)Using 3D TableTrix® microcarriers as a foundation, personalized gelatin microcarriers were designed in vitro, which self-assembled in vivo to form bone-cartilage organoids. These organoids were employ
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-07-04
- Views:189
[Introduction]

Recently, a collaborative research article titled "Dual-Functional In-Situ Self-Assembling Organoids for Bone-Cartilage Regeneration" has been jointly published in the journal Bioactive Materials [Impact Factor: 16.874]. The research was conducted by Professor Jianhao Colin and Professor Dan Xing from the Department of Orthopedics at Peking University People's Hospital, along with Professor Yanan Du from Tsinghua University. The study focuses on the development of dual-functional in-situ self-assembling organoids for the purpose of bone-cartilage regeneration.
Original url:http://www.sciencedirect.com/science/article/pii/S0012821X21005859
[Part 1 Research Background]
The osteochondral complex consists of articular cartilage, calcified cartilage, and subchondral bone, exhibiting intricate cartilage-bone interfaces and distinct tissue layer characteristics. In cases of bone-cartilage injuries, differences in the healing capacity and tissue integration of articular cartilage and bone pose challenges to achieving both structural and functional repairs of articular cartilage and subchondral bone. While researchers have developed scaffolds with biphasic, triphasic, multilayered, and gradient properties, achieving comprehensive success in animal models, there still exist limitations:
1.Biphasic scaffolds often fail to regenerate calcified cartilage and gradient tissue structures.
2.Triphasic and multilayered scaffolds often experience abrupt transitions between layers, making them susceptible to delamination and tissue separation under mechanical stress.
Organoid self-assembly has been utilized for drug screening, mechanistic studies, and investigating developmental or regenerative processes of various organ systems. The process of organoid self-assembly involves a series of cellular differentiation and self-patterning events, regulated by a variety of morphogens, such as transforming growth factor-β (TGF-β), epidermal growth factor (EGF), fibroblast growth factor (FGF), and gastrin, facilitated by growth factor-enriched culture media in 3D scaffolds.
However, current methods lack the ability to induce spatial patterning and developmental aspects of individual organoids through directed differentiation. A key limitation is the inadequacy of singular scaffolds and specific culture media to cultivate organoids with heterogeneous structures.
This study focuses on addressing the challenges of integrated repair in bone-cartilage injuries. Using hyaluronic acid (HA) and hydroxyapatite (HYP) modified 3D TableTrix® microcarriers developed by Huafan Biological Technology Co., Ltd. (referred to as Huafan Bio), bone-cartilage organoids were formed in situ through self-assembly within the body. These organoids were utilized to induce integrated regeneration of cartilage and bone.
[Part 2 Research Findings]
In the preliminary stages of this research, the research group found that 3D TableTrix® microcarriers loaded with MSCs can self-assemble in vitro to form functionalized 3D tissue blocks. This approach enhanced the stemness and paracrine activity of MSCs while mitigating cellular senescence.
To achieve bone-cartilage integrated regeneration and repair, the research group constructed personalized microcarriers. After loading with MSCs, these microcarriers were induced for chondrogenic and osteogenic differentiation for 7 days. Upon injection into bone-cartilage defects in beagle dogs, in-situ self-assembly and tissue repair of bone-cartilage organoids were achieved. These findings suggest that organoid technology holds promise as a regenerative therapeutic strategy for complex interface tissues.
[Part 3 Research Design]
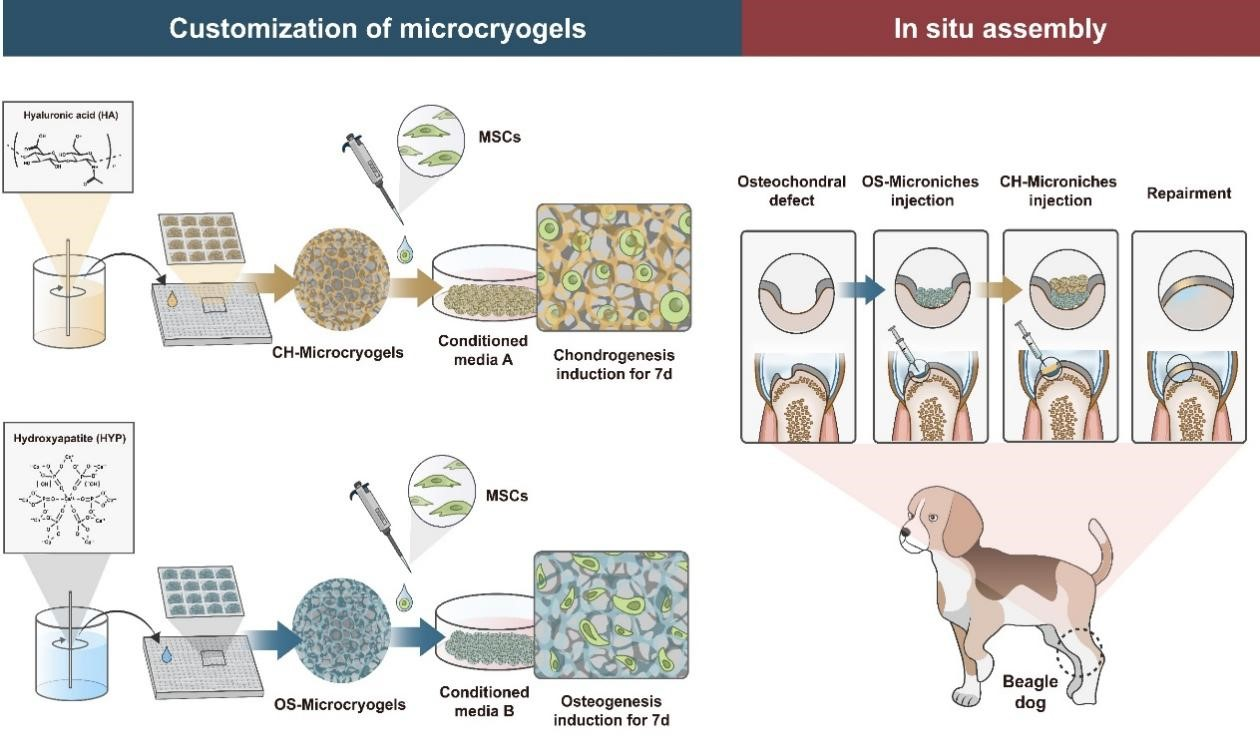
Figure 1: Conceptual Diagram of the Overall Research Design
The design of this study is illustrated in Figure 1. Based on the 3D TableTrix® microcarriers, personalized CH-microcarriers and OS-microcarriers were prepared using modifications with hyaluronic acid (HA) and hydroxyapatite (HYP). MSCs were seeded onto the microcarriers and then pre-cultured for 7 days in chondrogenic and osteogenic induction media, respectively. Bone-cartilage organoids were self-assembled in 3D molds through this pre-culturing process.
Pre-differentiated osteogenic and chondrogenic microcarriers were subsequently injected into bone-cartilage defects in the trochlear groove of beagle dogs. Postoperatively, samples were collected at 3 and 6 months to analyze the regenerative repair effects in all knee joints.
[Part 4 Research Content]
① Personalized Microcarriers with Favorable Physicochemical Properties and Biocompatibility
As seen in Figure 2, both CH-microcarriers and OS-microcarriers exhibited a porous and loose structure with average diameters of 217.10±68.35 μm and 98.16±29.94 μm, respectively. The water absorption rates were 14.85-fold and 6.82-fold for CH-microcarriers and OS-microcarriers, respectively. Viability staining results indicated excellent cell compatibility for all microcarriers. Notably, CH-microcarriers could float in PBS, while OS-microcarriers sank to the bottom.
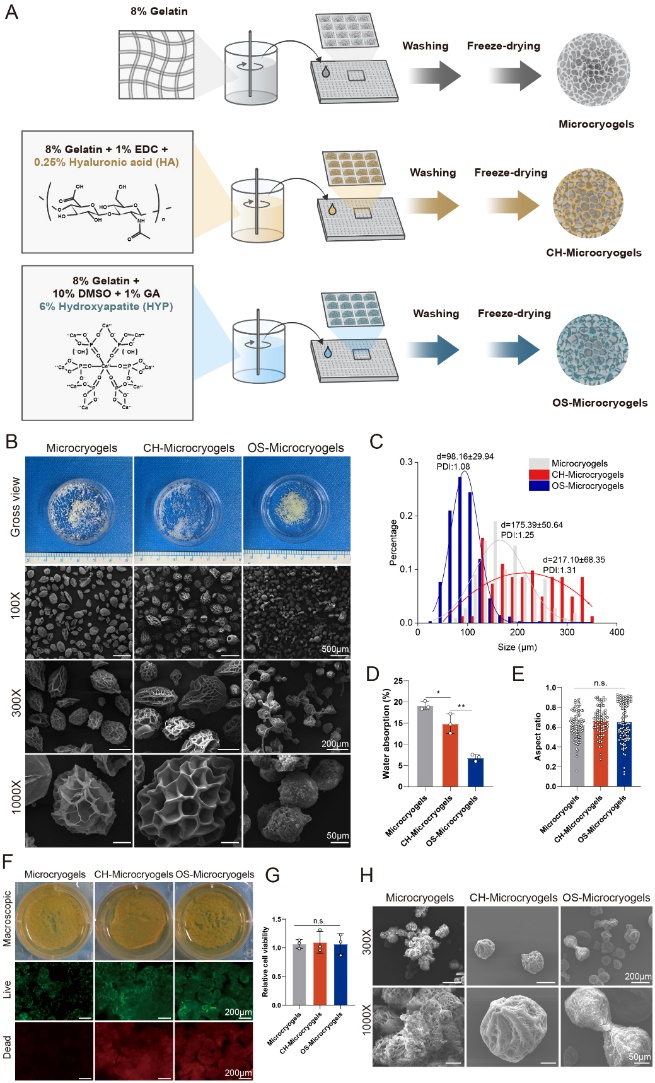
Figure 2: Preparation and Characterization of Personalized Microcarriers
Schematic representation of personalized microcarrier preparation (A),
Macroscopic and SEM images of different microcarriers (B)
Diameter distribution (C), • Water absorption rate (D)
Roundness analysis (E), • Live/dead staining (F)
Quantitative analysis of cell viability (G)
SEM image of MSC-loaded microcarriers after 14 days of culture (H)
② In Vitro Self-Assembly of Personalized Microcarriers into Bone-Cartilage Organoids
For the in vivo model study in this paper, a complete transverse spinal cord injury model was constructed in the 10th thoracic segment of rats (Figure 3A).
Combining behavioral assessment, magnetic resonance imaging, and histological analysis (Figure 3B-D), through comparison with different treatment methods, we found that gelatin microcarrier scaffolds loaded with MSCs could effectively promote regeneration and repair of rat spinal cord injuries (please refer to the original text for histological analysis images).
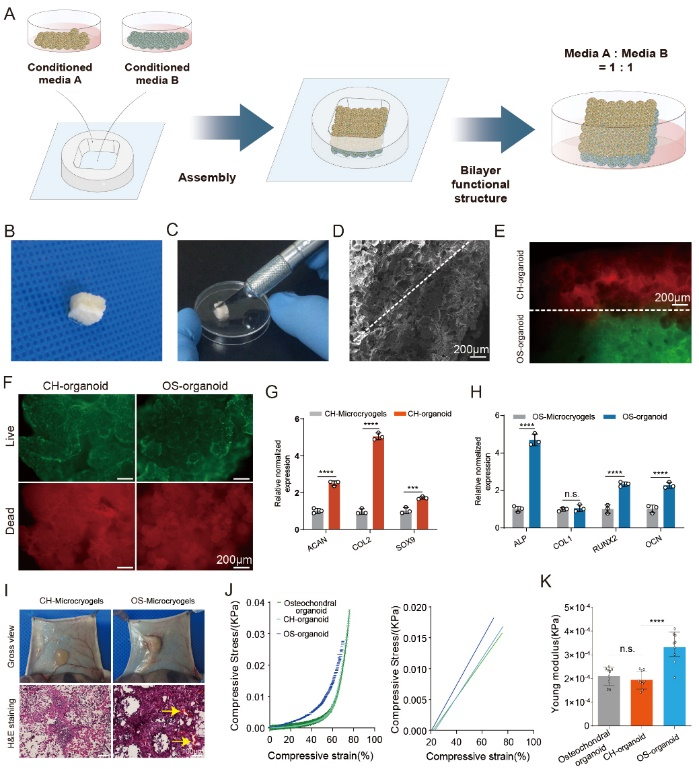
Figure 3: In Vitro Self-Assembly of Bone-Cartilage Organoids
Self-assembly process (A)
Macroscopic view of bone-cartilage organoids (B, C)
SEM results (D)
Cell tracing of self-assembled bone-cartilage organoids after 14 days of induction, red represents CH-microcarriers, green represents OS-microcarriers; live/dead staining of cartilage and bone portions of self-assembled bone-cartilage organoids (F)
Chondrogenic gene expression in CH-microcarriers and CH-organoids (cartilage layer of bone-cartilage organoids) (G) and relative osteogenic gene expression in OS-microcarriers and OS-organoids (bone layer of bone-cartilage organoids) (H)
Macroscopic view and H&E staining of CH-microcarriers and OS-microcarriers implanted subcutaneously in nude rats after 1 week (yellow arrows indicate neovascularization) (I)
Stress-strain curve (J) and Young's modulus (K) of bone-cartilage organoids and the components of CH-organoids and OS-organoids.
[Part 5 Research Conclusion]
Treatment of Bone-Cartilage Organoids in a Large Animal Model of Bone-Cartilage Injury
In the in vivo research model, a bone-cartilage defect model was established in the trochlear groove of beagle dogs (Figure 4). After 3 and 6 months of treatment, through the combination of imaging, macroscopic observation, and histopathological evaluation, comparisons of different treatment methods revealed that bone-cartilage self-assembling organoids achieved better regeneration, reconstruction, and integration of both cartilage and subchondral bone.
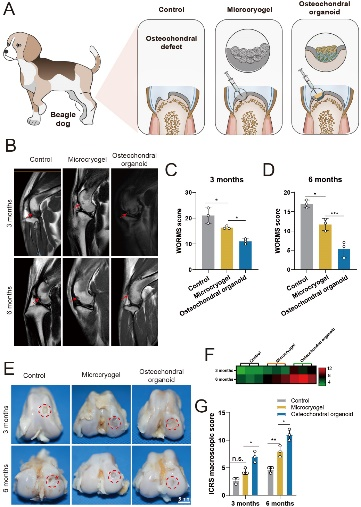
Figure 4: Experimental Groups for Repair of Beagle Dog Trochlear Groove Defects Using Self-Assembled Bone-Cartilage Organoids
[Research Application Materials]
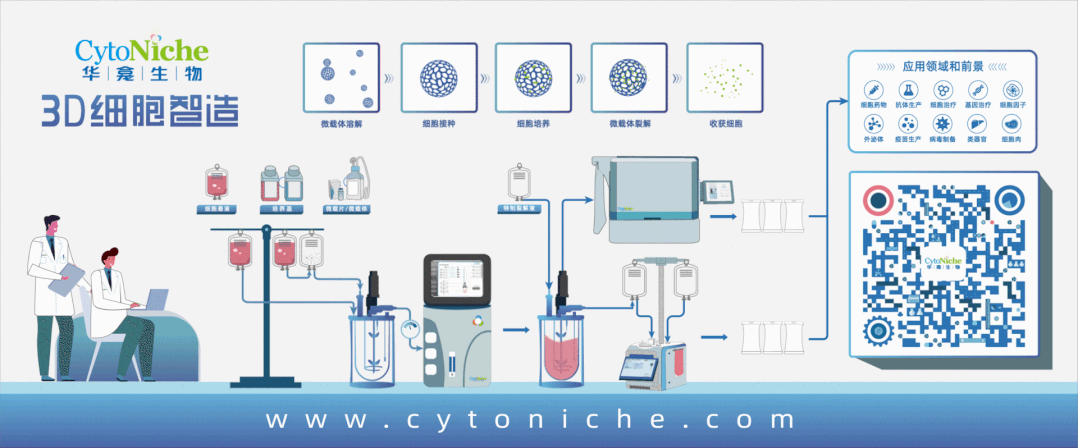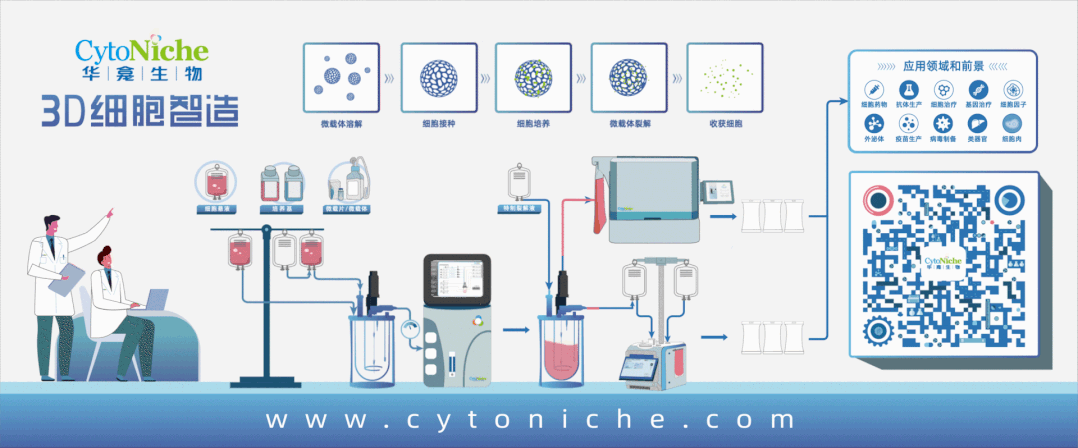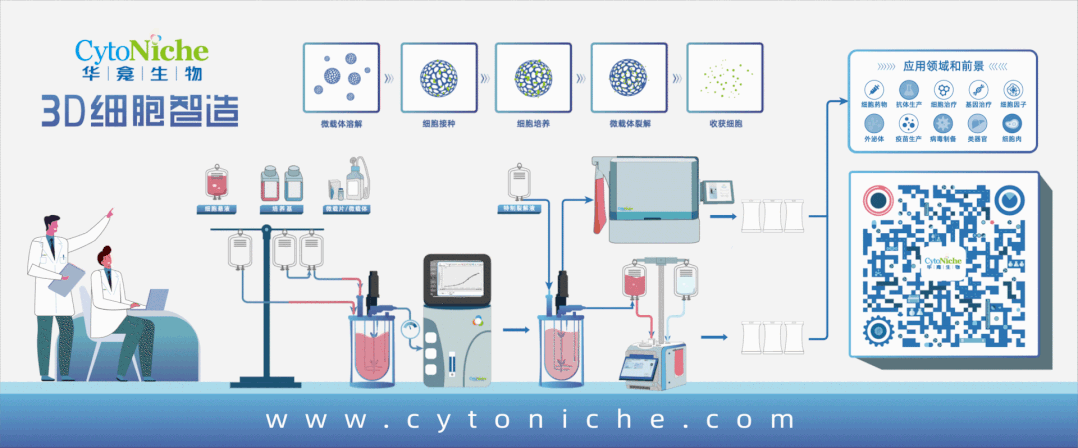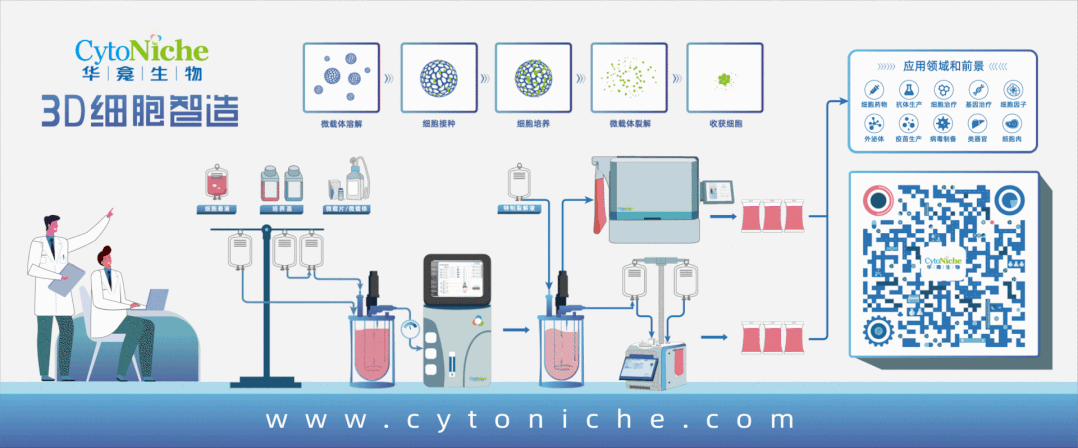
Core Product: 3D TableTrix® Microcarrier from CytoNiche
|
|
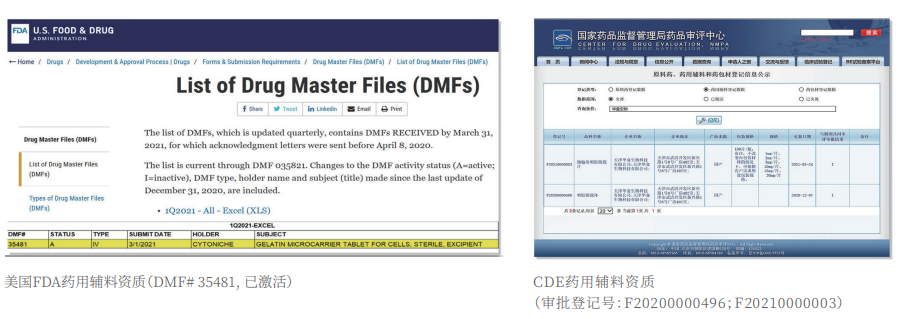 |
①Biomimetic: Composed of tens of thousands of elastic three-dimensional porous microcarriers, with a porosity >90%, controllable particle size in the range of 50-500μm, and uniformity ≤100μm, creating a truly biomimetic 3D culture environment.
②Certified: The product has obtained two qualifications as a drug excipient from the China Drug Evaluation (CDE), with registration numbers [F20200000496; F20210000003], and one qualification as an FDA Drug Master File (DMF) excipient, with registration number [DMF:35481].
3D FloTrix® Digest Solution
|
|
 |
3D FloTrix® Digest Solution
③ Easy Harvest: Specific degradation technology to digest microcarriers, enabling more efficient and gentle cell harvest compared to traditional methods.
Authoritative Testing Reports
④ Safer: Equipped with quality evaluation reports from authoritative institutions, including residual digestion detection, cell toxicity, pyrogen reaction, genetic toxicity, in vivo immunotoxicology, as well as evaluations for hemolysis, subcutaneous injection local irritation, systemic allergenicity, and peritoneal injection toxicity.
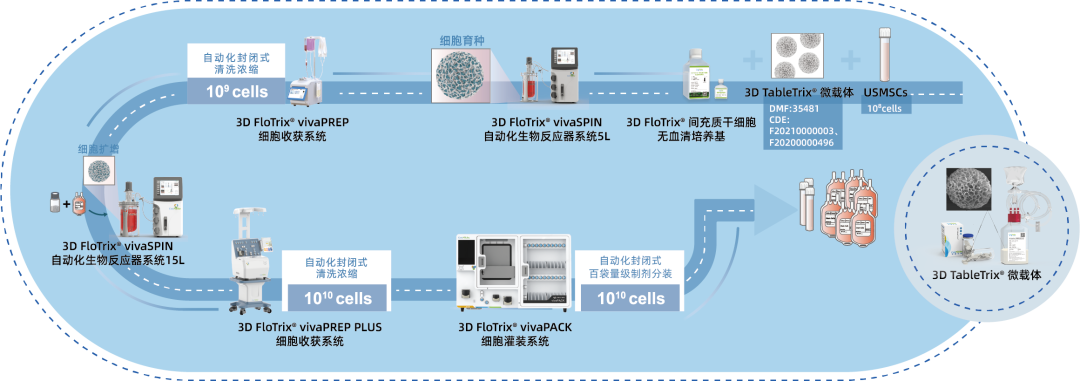
⑤ Scalable: By utilizing 3D culture techniques and combiningCytoNiche Bio's 3D Cell Intelligent Manufacturing Platform, large-scale cell culture can be achieved automatically in a closed system, allowing for the harvest of billions of cells.

If you have any questions or needs about this product, you can scan the code or call the hotline: 400-012-6688
We will ask technical experts to provide you with answers and help.
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP





 京公网安备 11010802037749号
京公网安备 11010802037749号
