【3D Microenvironment】Exploration of Modulating Stem Cell Function
- Categories:Company News
- Author:希泰希·巴克萨
- Origin:CytoNiche
- Time of issue:2023-05-25
- Views:173
(Summary description)Compared to traditional 2D microenvironments, the 3D microenvironment can better replicate key features and functions of the in vivo microenvironment for stem cell cultivation.
【3D Microenvironment】Exploration of Modulating Stem Cell Function
(Summary description)Compared to traditional 2D microenvironments, the 3D microenvironment can better replicate key features and functions of the in vivo microenvironment for stem cell cultivation.
- Categories:Company News
- Author:希泰希·巴克萨
- Origin:CytoNiche
- Time of issue:2023-05-25
- Views:173
[Part 1 Introduction]
01 Background Introduction
Tissues with a low number of endogenous cells lack the inherent ability to restore their mechanical strength and functionality, leading to clinical issues and medical burdens. Consequently, stem cell-based therapies offer significant clinical prospects for treating various tissue injuries. Tendon stem/progenitor cells (TSPCs) are a special group of stem cells found in tendons, possessing not only multipotency and self-renewal abilities but also exhibiting a tenogenic phenotype, making them ideal seed cells for tendon regeneration.
02 Bottleneck faced: 2D Traditional Cell Culture Model
Expanding a sufficient number of TSPCs for clinical therapy while preserving their stemness and tenogenic phenotype remains a major challenge. Commonly used 2D monolayer culture conditions for TSPCs fail to mimic the 3D physiological conditions, leading to the loss of tendon-related gene expression and a decline in regenerative capacity. Furthermore, the small growth surface of 2D culture dishes makes it impractical to produce stem cells safely and economically, and it does not meet the cell dosage requirements for future stem cell-based clinical therapies.
03 Solution: 3D Microenvironment Cell Culture Model
To better replicate key features and functions of the in vivo microenvironment for stem cell cultivation, the 3D microenvironment has garnered significant attention. 3D microenvironments are a novel stem cell culture approach, offering a small volume yet large surface area and ease of operation. Moreover, compared to traditional 2D microenvironments, they provide a biomimetic microenvironment, promoting substantial cell-cell and cell-ECM interactions in cell culture.
[Part 2 Literature Review]
In the current study, researchers employed CytoNiche's 3D gelatin microcarrier system to culture human tendon stem/progenitor cells (hTSPCs). They discovered that hTSPCs cultivated in the 3D gelatin microcarrier system exhibited outstanding stem cell characteristics and functionality, both in vitro and in vivo, surpassing those cultured using traditional 2D methods. Furthermore, the researchers delved deeper into exploring the underlying mechanisms behind these findings.
01 Materials and Methods:
The researchers utilized Hua Kang Biological's 3D TableTrix® microcarrier system as the 3D microenvironment for cell culture. They further assessed the cell viability and proliferation capacity, differentiation ability, flow cytometry analysis, immunofluorescence staining, and transcriptome analysis of the cells expanded in the 3D microenvironment. Additionally, animal experiments were conducted to characterize the tendon formed in vivo.
02 Experimental Results:
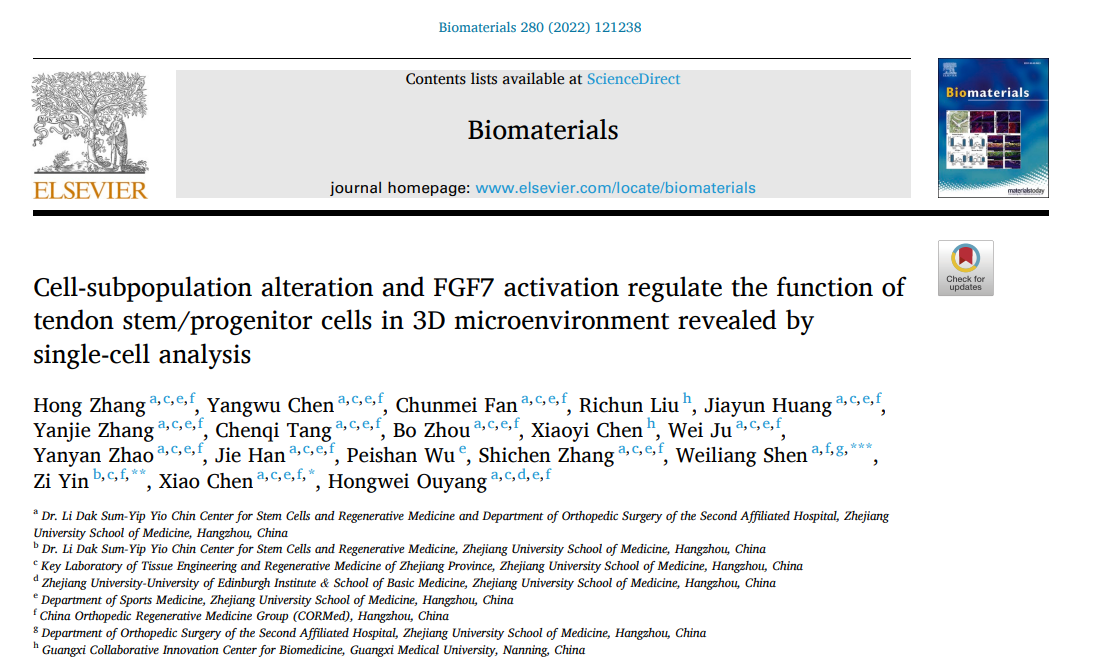
The 3D microcarrier system provided an excellent platform for hTSPC culture.
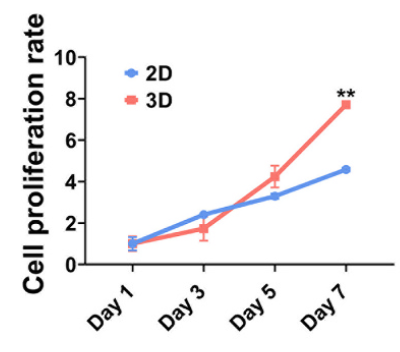
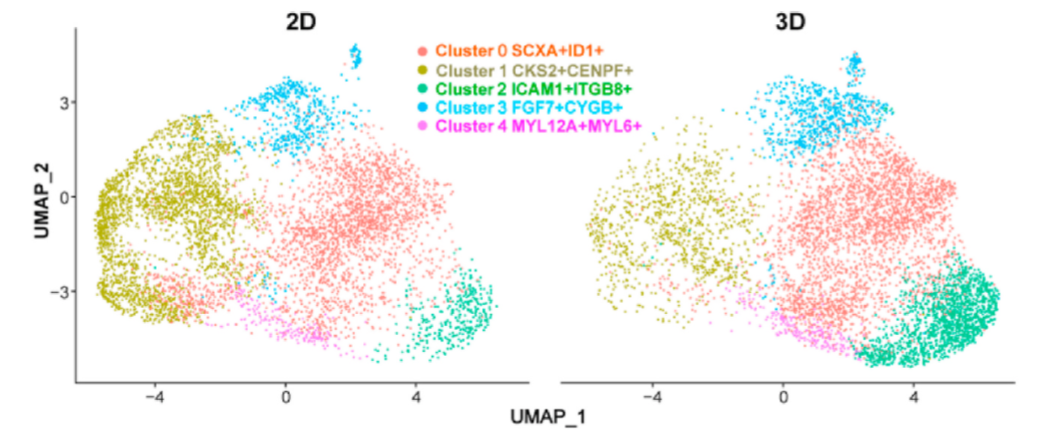
Figure 1: Cell Proliferation Multiplication Curve and Live/Dead Staining Fluorescence Images.
hTSPCs in the 3D group tend to exhibit characteristics more akin to young stem cells rather than aging stem cells: Immunofluorescence staining reveals that compared to the 2D group, hTSPCs in the 3D group display a less spread-out morphology, characterized by smaller cell area and volume, improved nuclear-to-cytoplasm ratio, and increased sphericity.
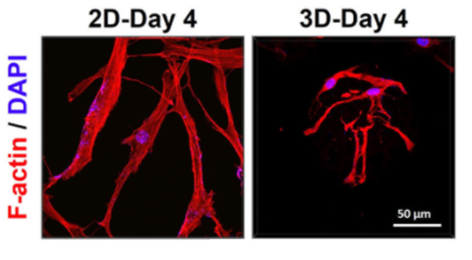
Figure 2: 3D Confocal Images of hTSPCs after Immunofluorescence Staining.
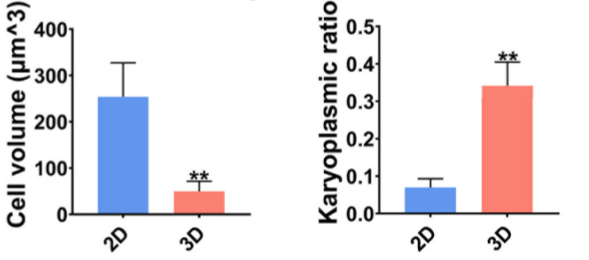
Figure 3: Cell Volume (left) and Nuclear-to-Cytoplasm Ratio (right) of hTSPCs in 2D and 3D Cultures after 4 Days of Cultivation.
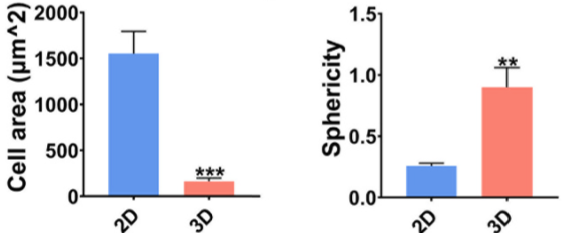
Figure 4: Cell Area (left) and Sphericity (right) of hTSPCs in 2D and 3D Cultures after 4 Days of Cultivation.
The hTSPCs expanded in the 3D microcarrier system exhibited enhanced tenogenic differentiation ability.
● They showed increased expression of tendon-related genes and higher levels of tendon-specific markers compared to the 2D culture method.
Immunofluorescence staining confirmed a significant upregulation in the expression of tendon-specific markers in the 3D group.
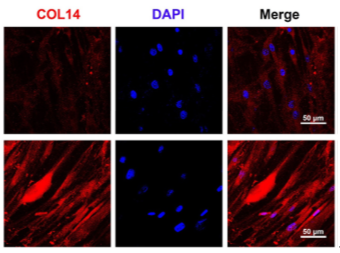
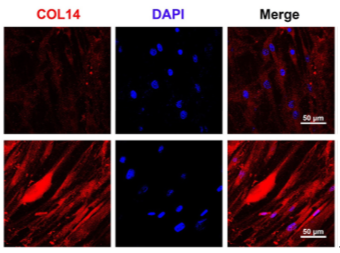
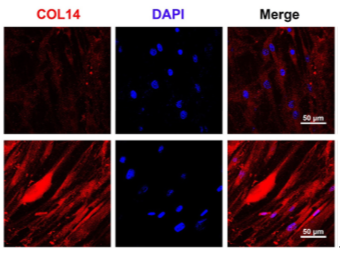
Figure 5: Immunofluorescence Staining of TNMD, COL1, and COL14 after 7 days of Tendon Induction.
The expression of tendon-related genes was significantly higher in the 3D microenvironment.
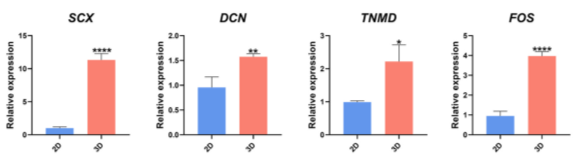
Figure 6: qRT-PCR Analysis of SCX, FOS, DCN, and TNMD in hTSPCs after 4 Days of Cultivation.
● 3D cultivation of hTSPCs in a new cellular microenvironment contributes to enhanced tenogenic capability.
Researchers compared the proportions of each subpopulation in the 3D and 2D groups. While the CKS2+CENPF+ subpopulation was significantly enriched in the 2D group, other clusters were more abundant in the 3D group. Specifically, the ICAM1+ITGB8+FGF7+ and FGF7+CYGB+ subpopulations predominated in the 3D group. Within the ICAM1+ITGB8+FGF7+ and FGF7+CYGB+ subpopulations, the expression of tendon-related genes increased, while it was lower in the CKS2+CENPF+ subpopulation.
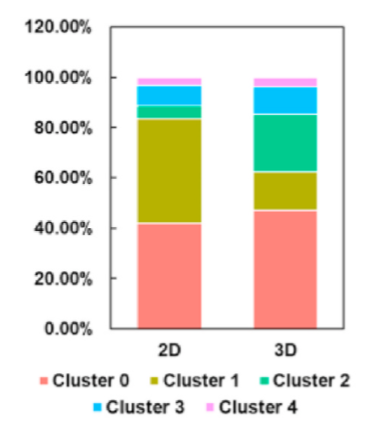

Figure 7: UMAP plot dividing hTSPCs into five subpopulations (left) and the corresponding proportion bar chart (right).
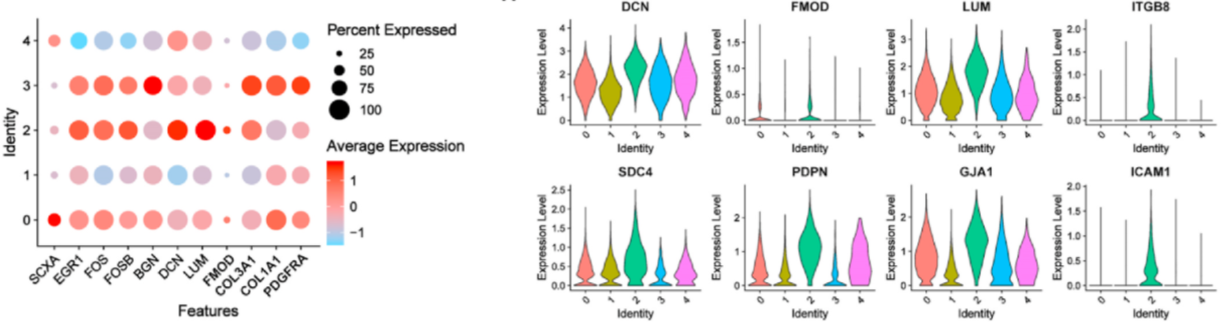
Figure 8: Heatmap and Violin Plots of Gene Expression.
● The activation of FGF7 signaling in the 3D microenvironment enhances the tenogenic capability of hTSPCs through cell-cell interactions.
Researchers found that the 3D microenvironment enhances the tenogenic phenotype by activating FGF7. Integrin-mediated FGF7 signaling regulates the ERK MAPK/PI3K/STAT pathway in the ICAM1+ITGB8+FGF7+ and FGF7+CYGB+ subpopulations, thus enhancing the fibrotic phenotype of hTSPCs in the 3D microenvironment through cell-cell interactions.
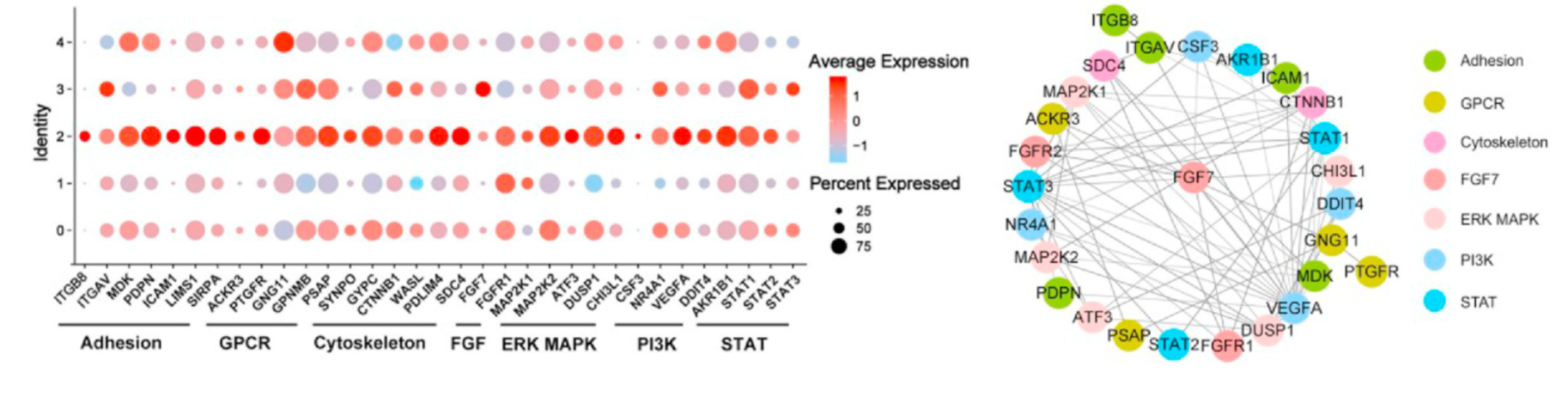
Figure 9: Expression of Genes Related to Integrin-FGF7-ERK MAPK/PI3K/STAT Signaling (left) and Gene Interaction
Network of Integrin-FGF7-ERK MAPK/PI3K/STAT Signaling in the ICAM1+ITGB8+FGF7+ Subpopulation (right).
hTSPCs expanded using the 3D microcarrier system exhibited superior in vivo tendon formation capability. Researchers conducted animal experiments and performed relevant characterization tests at two weeks and four weeks post-surgery.
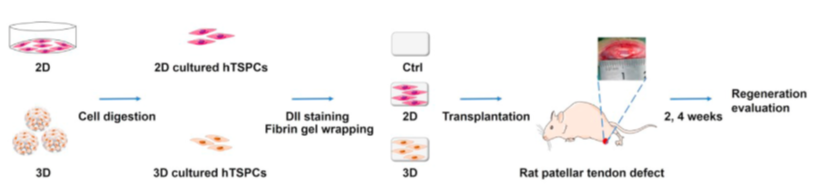
Figure 10: Schematic Illustration of the Animal Experiment.
At two weeks post-surgery, the 3D group exhibited dense and well-organized regenerative tendons at the repair site, with minimal inflammation and swelling observed in the macroscopic morphology. In contrast, the 2D group and the control group showed loosely arranged soft tissues with round-shaped cells and some blood vessels, accompanied by noticeable inflammation and swelling. The collagen fibers in the 3D group were larger and more organized compared to the 2D group and the control group.

Figure 11: Representative Images of Rat Patellar Tendon Repair at Two Weeks Post-Surgery stained with H&E, Masson's Trichrome, and Polarized Light.
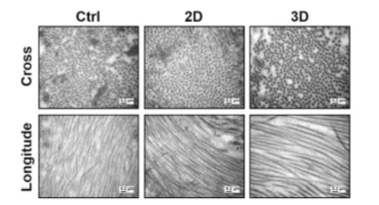
Figure 12: TEM Images of Cross-sectional and Longitudinal Views of Collagen Fibers in Tendon Tissue at Two Weeks Post-Surgery.
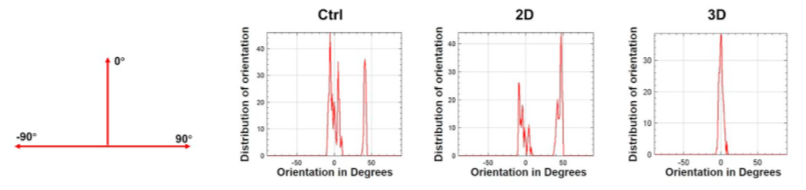
Figure 14: Distribution of Collagen Fiber Orientation Angles at Two Weeks Post-Surgery.
At four weeks post-surgery, compared to the tendon at two weeks, the repaired tendon in the 3D group appeared denser with more spindle-shaped cells distributed along the axis of tensile load. There was less inflammation observed in the macroscopic morphology. On the other hand, the regenerated tendons in the 2D group and the control group were filled with multiple cells and disordered collagen fibers, accompanied by some inflammation and swelling.
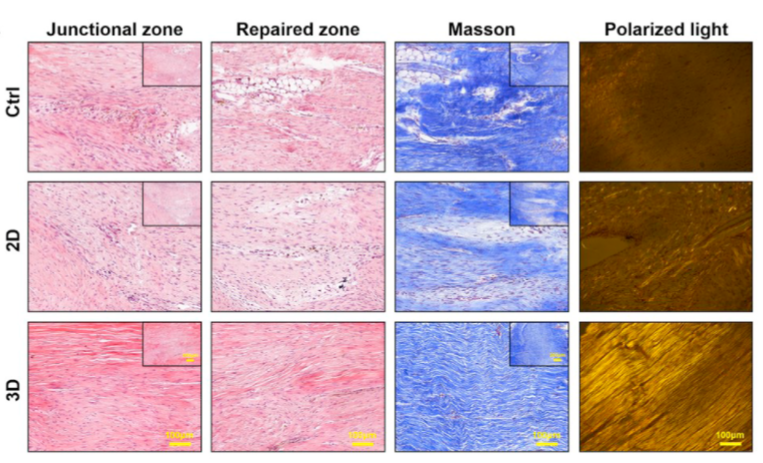
Figure 15: Representative Images of Repaired Tendons at Four Weeks Post-Surgery stained with H&E, Masson's Trichrome, and Polarized Light.
In the T2-weighted magnetic resonance imaging (MRI) images, the regenerated tendons in the 3D group exhibited low-intensity, uniform signals, and clear edges, similar to healthy tendons. In contrast, the regenerated tendons in the 2D group and the control group showed irregular contours and heterogeneous high-intensity signals, indicating that injury and inflammation were still present in these two groups.
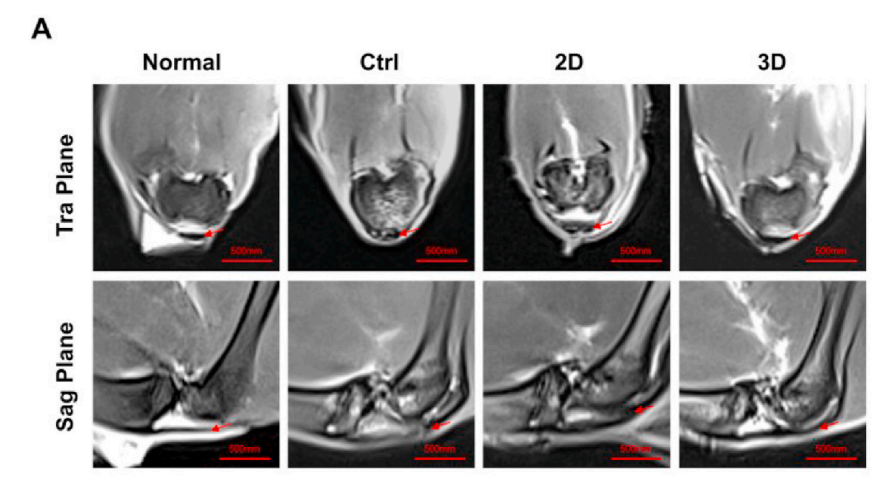
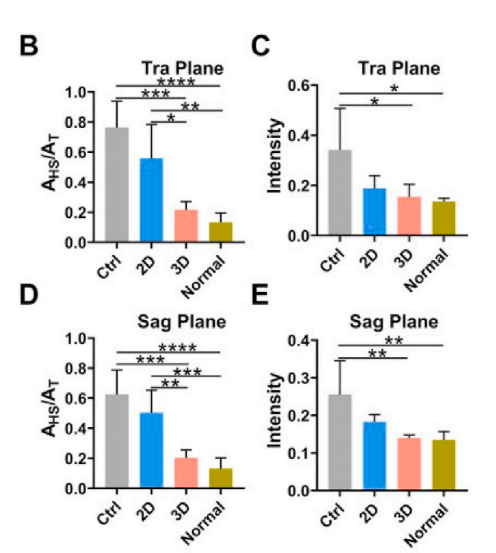
Figure 16: T2-weighted MRI scans of repaired patellar tendons and their semi-quantitative MRI images (A), analysis of the ratio of high or moderate signal areas (AHS) to total longitudinal area (AT) (B, D), and analysis of the tendon lesion intensity (C, E). Red arrows indicate the tendon area.
Compared to the 2D and control groups, the 3D group exhibited superior biomechanical properties. The 3D group demonstrated significantly higher stiffness, stress at failure, and ultimate load compared to the other groups. The modulus of the 3D, 2D, and control groups was 42.74%, 14.05%, and 14.24% of the normal rat patellar tendon, respectively. The energy absorbed at failure in the 3D group was 2.53 times higher than that of the 2D group and 3.06 times higher than that of the control group. There were no significant differences in the mechanical properties between the 2D and control groups.
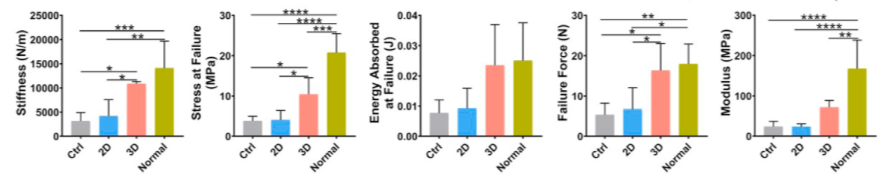
Figure 17: Mechanical Properties of Regenerated Patellar Tendon at Four Weeks Post-Surgery: Stiffness | Stress at Failure | Energy Absorbed at Failure | Ultimate Load | Modulus.
Conclusion:
This study explored the regulatory mechanisms of hTSPCs' functionality in a 3D microenvironment and identified crucial changes in cell subpopulations and the activation of FGF7, which are essential for enhancing the tenogenic capability of hTSPCs.
Using the 3D TableTrix® microcarrier as the 3D microenvironment for hTSPC expansion resulted in improved cell viability, proliferation rate, and younger cell population, as well as enhanced stemness. In vivo experiments also demonstrated superior biological efficacy of hTSPCs cultivated in the 3D microenvironment.
Reference:
Hong Zhang, Yangwu Chen, Chunmei Fan, et al. Cell-subpopulation alteration and FGF7 activation regulate the function of tendon stem/progenitor cells in 3D microenvironment revealed by single-cell analysis. Biomaterials. 2022 Jan;280:121238.
Core Product: 3D TableTrix® Microcarrier from CytoNiche
|
|
 |
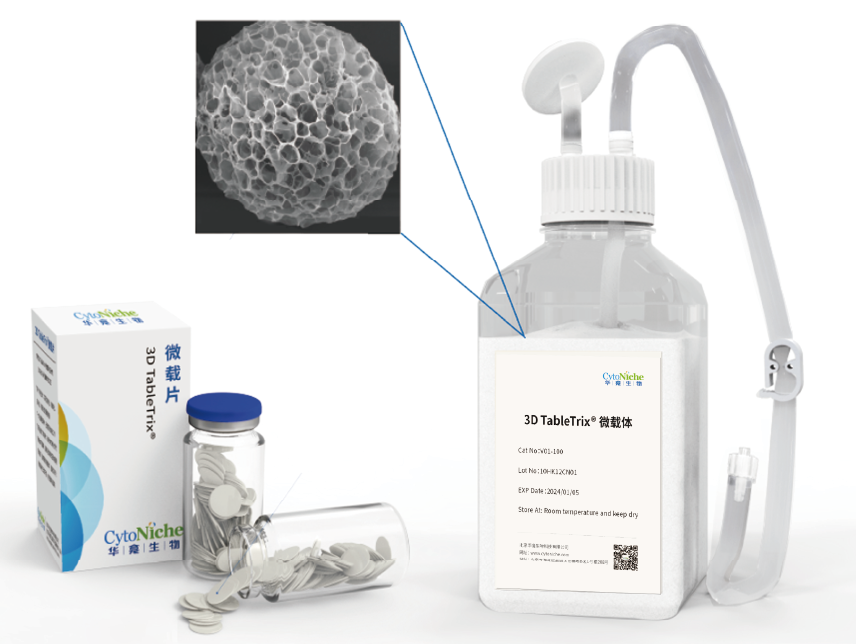
①Biomimetic: Composed of tens of thousands of elastic three-dimensional porous microcarriers, with a porosity >90%, controllable particle size in the range of 50-500μm, and uniformity ≤100μm, creating a truly biomimetic 3D culture environment.
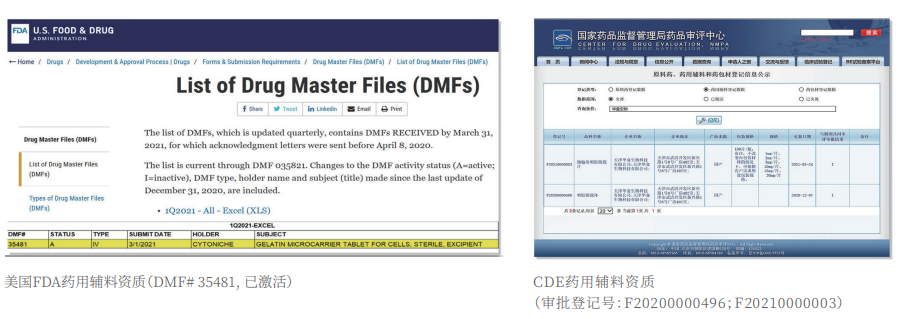
②Certified: The product has obtained two qualifications as a drug excipient from the China Drug Evaluation (CDE), with registration numbers [F20200000496; F20210000003], and one qualification as an FDA Drug Master File (DMF) excipient, with registration number [DMF:35481].
3D FloTrix® Digest Solution
|
|
 |
3D FloTrix® Digest Solution
③ Easy Harvest: Specific degradation technology to digest microcarriers, enabling more efficient and gentle cell harvest compared to traditional methods.
Authoritative Testing Reports
④ Safer: Equipped with quality evaluation reports from authoritative institutions, including residual digestion detection, cell toxicity, pyrogen reaction, genetic toxicity, in vivo immunotoxicology, as well as evaluations for hemolysis, subcutaneous injection local irritation, systemic allergenicity, and peritoneal injection toxicity.
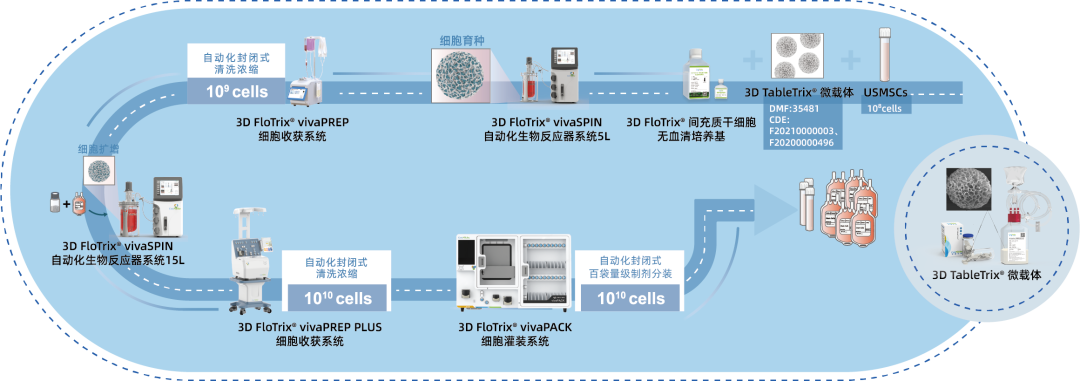
⑤ Scalable: By utilizing 3D culture techniques and combiningCytoNiche Bio's 3D Cell Intelligent Manufacturing Platform, large-scale cell culture can be achieved automatically in a closed system, allowing for the harvest of billions of cells.

If you have any questions or needs about this product, you can scan the code or call the hotline: 400-012-6688
We will ask technical experts to provide you with answers and help.
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier, is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP




 京公网安备 11010802037749号
京公网安备 11010802037749号
