Frontier Research: [3D Microcarrier] Facilitates Efficacy Verification and Mechanism Exploration in Spinal Cord Injury Regeneration and Repair
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-06-08
- Views:173
(Summary description)Abstract: The application of 3D TableTrix® microcarriers and their derivative technologies holds promise for accelerating the translation and promotion of tissue engineering techniques in the field of
Frontier Research: [3D Microcarrier] Facilitates Efficacy Verification and Mechanism Exploration in Spinal Cord Injury Regeneration and Repair
(Summary description)Abstract: The application of 3D TableTrix® microcarriers and their derivative technologies holds promise for accelerating the translation and promotion of tissue engineering techniques in the field of
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-06-08
- Views:173

Recently, a collaborative research effort by Professor Bin Wang's team from the First Affiliated Hospital of Zhejiang University, Professor Duanan Du's team from Tsinghua University, and Professor Bin Zhao's team from the Second Affiliated Hospital of Shanxi Medical University, published an article titled "Self-assembly of gelatin microcarrier-based MSC microtissues for spinal cord injury repair" in the Chemical Engineering Journal (Impact Factor: 16.744). The study explores the application of "self-assembly" gelatin microcarrier scaffolds loaded with mesenchymal stem cells for spinal cord injury repair.
URL: https://doi.org/10.1016/j.cej.2022.138806.
[Research Findings]
The gelatin microcarrier loaded with mesenchymal stem cells (MSC) (developed by Beijing CytoNiche Biotechnology Co., Ltd., hereinafter referred to as CytoNiche Biotechnology) can undergo "self-assembly" and form microtissue blocks with a certain spatial architecture during in vitro culture. This novel approach addresses a series of challenges faced by traditional tissue engineering materials and cell transplantation therapy for spinal cord injury (SCI), such as effectively improving MSC cell viability and paracrine function. Ultimately, it aims to promote effective regeneration and repair of spinal cord injuries. The study also conducted transcriptomic gene sequencing analysis to explore the potential repair mechanisms of MSC microtissue blocks applied in spinal cord injury treatment.
[Research Background]
Spinal cord injury (SCI) is a severe central nervous system disorder that poses a significant threat to human life and health. It is characterized by sensory and motor impairments of the limbs, neuropathic pain, and complications involving respiratory, urinary, and other systems. Pathological features include axonal damage, primary or secondary tissue edema, and necrosis. Epidemiological surveys have shown that there are up to 770,000 new cases of spinal cord injuries worldwide each year, with the majority affecting young and middle-aged males. This imposes a substantial burden on society.
Due to the complex pathogenesis of spinal cord injuries, conventional treatments such as drug therapy and surgery often have limited efficacy. In recent years, researchers have increasingly focused on the combination of tissue engineering materials and cell transplantation for spinal cord injury treatment. Tissue engineering materials can provide certain protection and promotion effects for cells to exert biological effects, such as protecting cells from damage in the inflammatory microenvironment of the host and promoting better differentiation of cells into functional cells.
After spinal cord injury occurs, the extent of tissue damage varies, and different tissue engineering materials have different sources and preparation processes, leading to changes in their physical parameters and structural characteristics. Therefore, designing ideal biomaterials to achieve integration with the host tissue has become one of the key research areas in the field of tissue engineering materials.
Since 2014, Professor Duanan Du's team from Tsinghua University School of Medicine has been pioneering the use of 3D porous microcarriers combined with stem cells for the treatment of related diseases. They have achieved a series of research results in various fields, including diabetes, lower limb ischemic injuries, osteoarthritis, and liver fibrosis. This study marks the first application of this microcarrier scaffold in the treatment of central nervous system injuries.
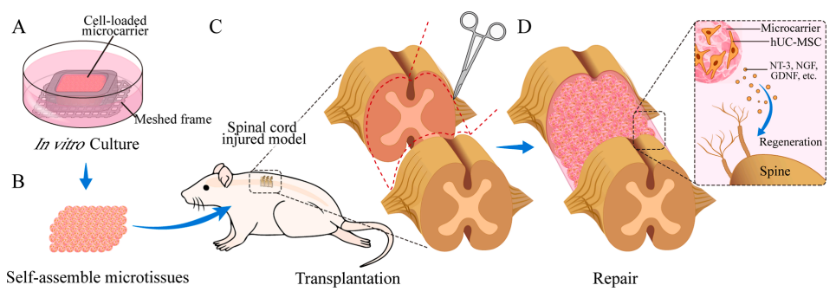
Schematic diagram of overall research design
① Formation of Microtissue Blocks by Culturing MSCs on Gelatin Microcarrier Scaffold
The study found that when MSCs were seeded onto the CytoNiche Biotechnology 3D TableTrix® gelatin microcarrier scaffold and cultured in vitro within a pre-printed mold, they formed microtissue blocks with specific characteristics over a period of time.
During the formation of microtissue blocks, the MSCs inside exhibited higher cell viability and delayed cellular senescence, with minimal apoptosis (Figure 1A-F). Laser confocal microscopy was used to reconstruct and observe the microstructure of the microtissue blocks (Figure 2). Additionally, the paracrine function of MSCs within the microtissue blocks was improved; they secreted higher levels of neurotrophic factors such as NT-3, NGF, and GDNF (Figure 1G-H). These findings provide a solid foundation for the transplantation of microtissue blocks into the host for therapeutic purposes.
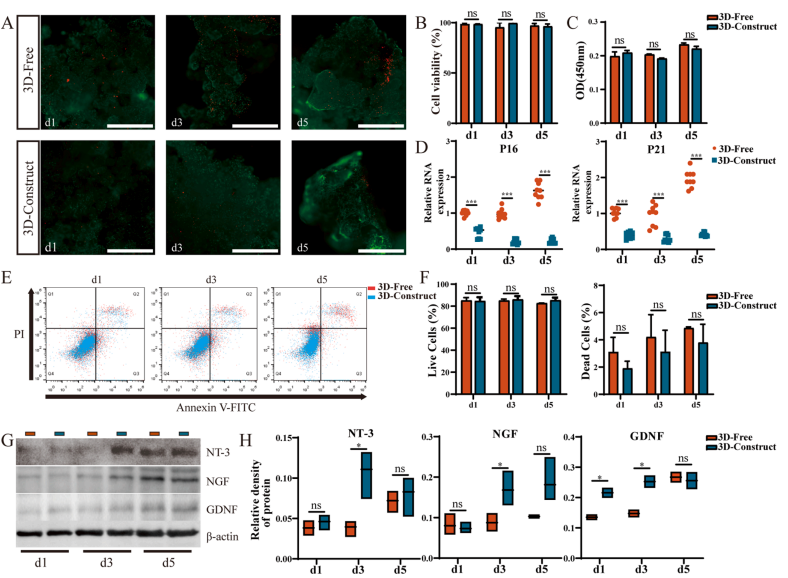
Figure 1: In vitro validation of loaded MSC micro tissue blocks
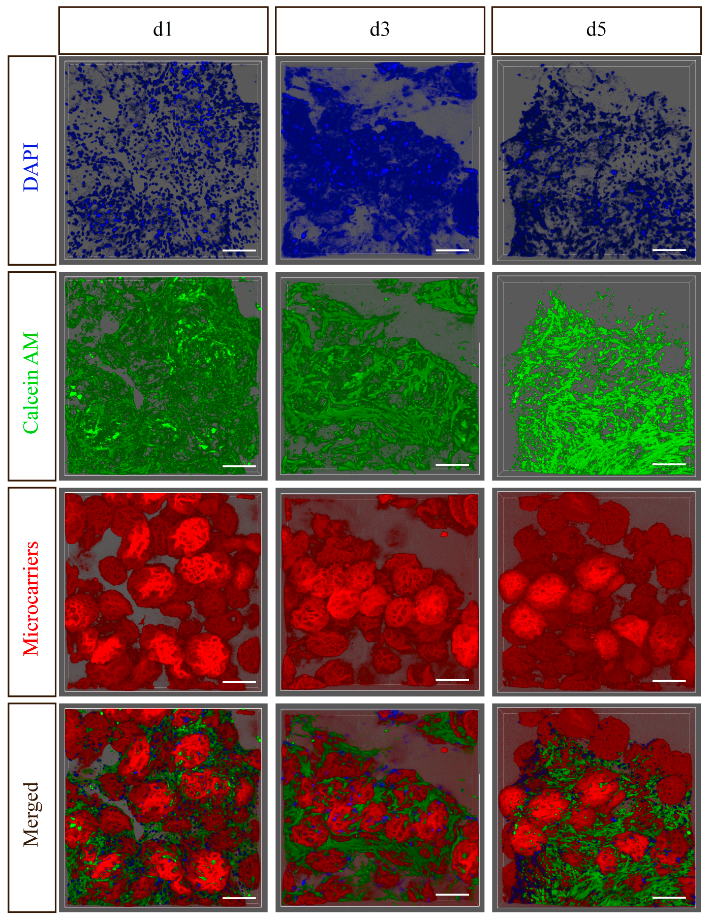
Figure 2: Laser confocal scanning results of microstructure blocks loaded with MSC (Scale bar=200)μM)
② Treatment of Rat Spinal Cord Injury Model with MSC Microtissue Blocks
In the in vivo model study, a complete transection injury of the thoracic segment 10 of the rat spinal cord was created (Figure 3A).
Combining behavioral assessments, magnetic resonance imaging (MRI), and histological analysis (Figure 3B-D), bycomparing different treatment methods, the researchers found that the gelatin microcarrier scaffold loaded with MSC microtissue blocks effectively promoted the regeneration and repair of rat spinal cord injuries. (Please refer to the original article for images from histological analysis.)
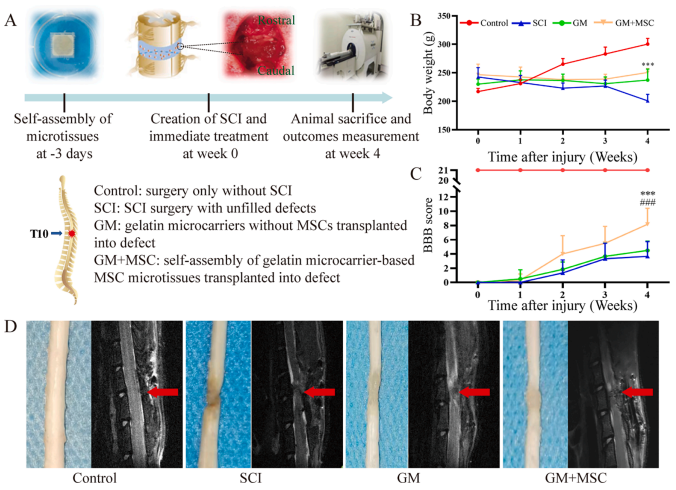
Figure 3: Preparation and evaluation of in vivo animal models
③ Mechanisms of MSC Microtissue Blocks in Promoting Spinal Cord Injury Repair
In this study, spinal cord tissue samples were collected from the spinal cord injury group and the group treated with MSC microtissue blocks for transcriptome sequencing analysis. The results showed that compared to the spinal cord injury group, there were 32 upregulated genes and 163 downregulated genes in the spinal cord tissue after treatment with MSC microtissue blocks.
By combining Gene Ontology (GO) functional enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis, it was revealed that the differentially expressed genes were mainly related to the regulation of internal inflammatory immune responses, while the upregulated genes were mostly associated with the structural remodeling of neural tissues (for more detailed analysis, please refer to the original article).
These findings provide valuable insights into the therapeutic effects of MSC microtissue blocks in spinal cord injury repair.
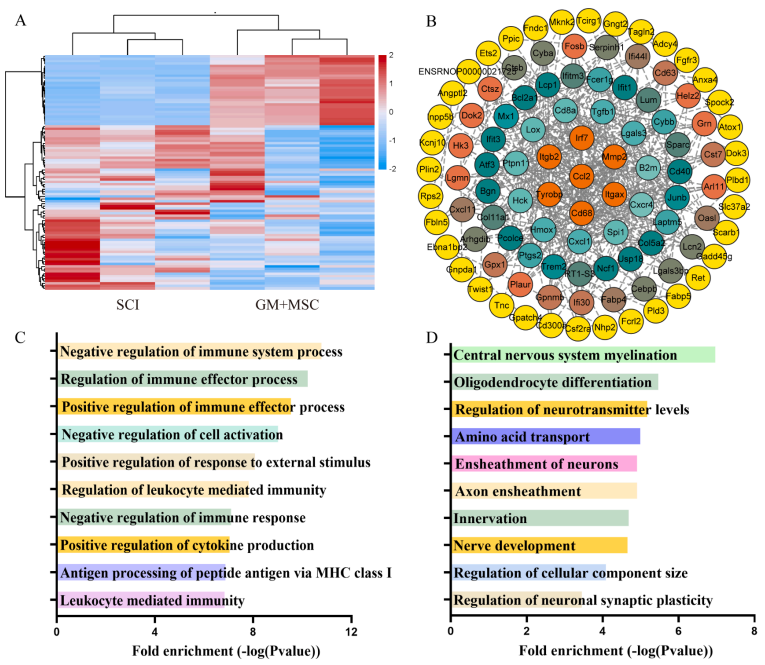
Figure 4: Transcriptome sequencing analysis results of rat spinal cord tissue
[Research Conclusion]
This study successfully constructed a gelatin microcarrier-based scaffold loaded with mesenchymal stem cells (MSCs) using the "self-assembly" property. It effectively maintained the cell viability and improved the biological functions of the cells. Furthermore, the therapeutic effects of the scaffold were validated in an in vivo animal model of spinal cord injury repair, providing valuable insights for the clinical treatment of spinal cord injuries.
Moreover, through transcriptomic analysis, the potential mechanisms of action for the above treatment were revealed, laying a solid theoretical foundation for subsequent related research. Therefore, the application of 3D TableTrix® microcarriers and its derivative technologies is expected to accelerate the translation and promotion of tissue engineering in the field of spinal cord injury repair.
[Research Team]
Professor Bin Wang from Zhejiang University, Professor Duanan Du from Tsinghua University School of Medicine, and Professor Bin Zhao from the Second Affiliated Hospital of Shanxi Medical University are the corresponding authors of this paper. Dr. Haifeng Liu from Professor Zhao's and Professor Wang's research groups (2022 full-time doctoral student at Shanxi Medical University) and Dr. Xiaojun Yan, Co-founder & CTO of CytoNiche Bio, were the co-first authors of this paper (Ph.D. student in Professor Du's research group).
[Research Application Materials]
Core Product: 3D TableTrix® Microcarrier from CytoNiche
|
|
 |
①Biomimetic: Composed of tens of thousands of elastic three-dimensional porous microcarriers, with a porosity >90%, controllable particle size in the range of 50-500μm, and uniformity ≤100μm, creating a truly biomimetic 3D culture environment.
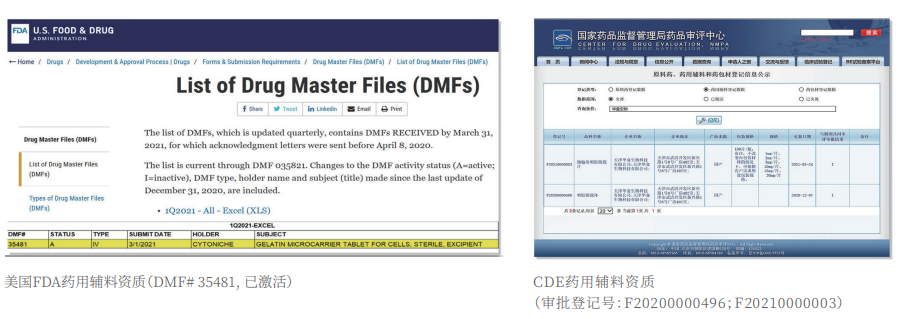
②Certified: The product has obtained two qualifications as a drug excipient from the China Drug Evaluation (CDE), with registration numbers [F20200000496; F20210000003], and one qualification as an FDA Drug Master File (DMF) excipient, with registration number [DMF:35481].
3D FloTrix® Digest Solution
|
|
 |
3D FloTrix® Digest Solution
③ Easy Harvest: Specific degradation technology to digest microcarriers, enabling more efficient and gentle cell harvest compared to traditional methods.
Authoritative Testing Reports
④ Safer: Equipped with quality evaluation reports from authoritative institutions, including residual digestion detection, cell toxicity, pyrogen reaction, genetic toxicity, in vivo immunotoxicology, as well as evaluations for hemolysis, subcutaneous injection local irritation, systemic allergenicity, and peritoneal injection toxicity.
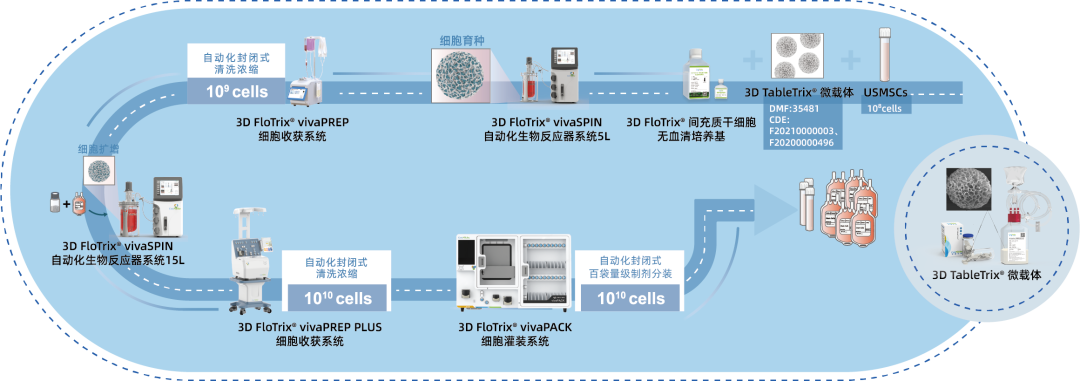
⑤ Scalable: By utilizing 3D culture techniques and combiningCytoNiche Bio's 3D Cell Intelligent Manufacturing Platform, large-scale cell culture can be achieved automatically in a closed system, allowing for the harvest of billions of cells.

If you have any questions or needs about this product, you can scan the code or call the hotline: 400-012-6688
We will ask technical experts to provide you with answers and help.
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier, is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP





 京公网安备 11010802037749号
京公网安备 11010802037749号
