Opportunities and Challenges of Downstream Separation Technologies for MSC Cells Based on Microcarrier Culture
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-04-06
- Views:36
(Summary description)Although cell culture technologies have become more mature, there are still many challenges in the downstream processing steps, including harvesting, filtration, and volume concentration.
Opportunities and Challenges of Downstream Separation Technologies for MSC Cells Based on Microcarrier Culture
(Summary description)Although cell culture technologies have become more mature, there are still many challenges in the downstream processing steps, including harvesting, filtration, and volume concentration.
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-04-06
- Views:36
[Foreword]
Mesenchymal stem cells (MSCs) have the potential for multi-lineage differentiation and are easily collected, isolated, and grown in vitro, making them promising in the field of regenerative medicine. Since MSCs are adherent cells, the addition of microcarriers to the culture system is required to increase the surface area for cell attachment and growth. Although microcarrier-based MSC culture technology has become relatively mature, there are still many challenges in the downstream processing steps, including harvesting, filtration, and volume concentration. Recently, a team led by Professor Michael Kallos from the University of Calgary, Canada, published a review in the journal Biotechnology & Bioengineering, assessing the current status of downstream separation technologies and proposing a decision flowchart.
Cell harvesting is the first downstream processing stage, which involves separating the cells from the microcarriers.
Microcarriers can be roughly divided into conventional microcarriers and responsive microcarriers:
▷ For cells cultured on conventional microcarriers, the addition of proteases to degrade the cell-matrix is usually required during harvesting to release the cells. However, proteases may cause damage to the cells, affecting their viability and proliferation
▷ The separation of cells from responsive microcarriers requires the addition of specific stimuli to alter the microcarriers themselves, leading to the detachment of cells. However, some stimuli still cause damage to the cells, and prolonged processing time also affects the viability of harvested cells.
In this review, the authors list the effects of different types of microcarriers, various proteases, and stimuli, as well as their impact on cell viability (Figure 1, Figure 2).
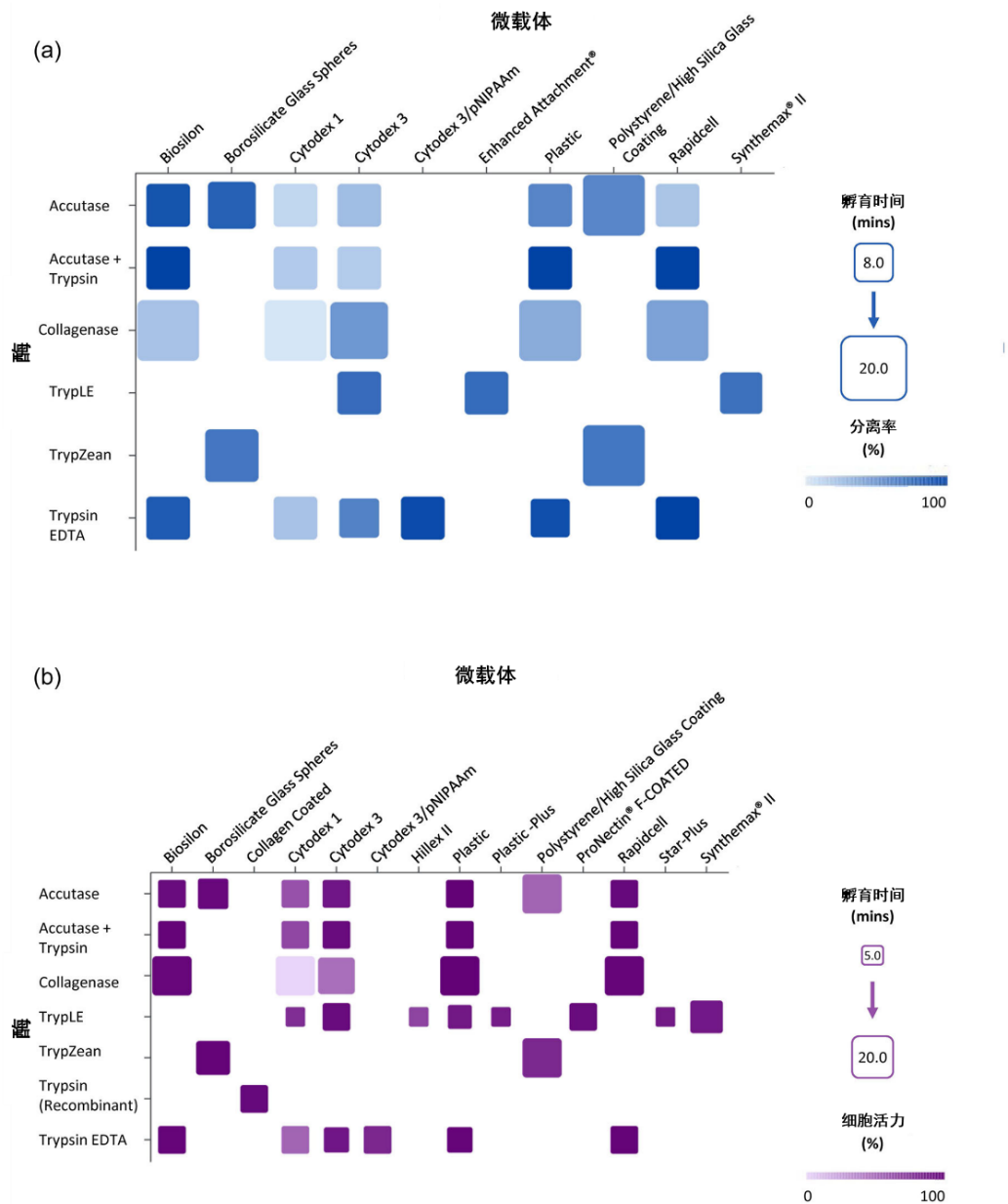
Figure 1: Comparison of different combinations of proteases and microcarriers
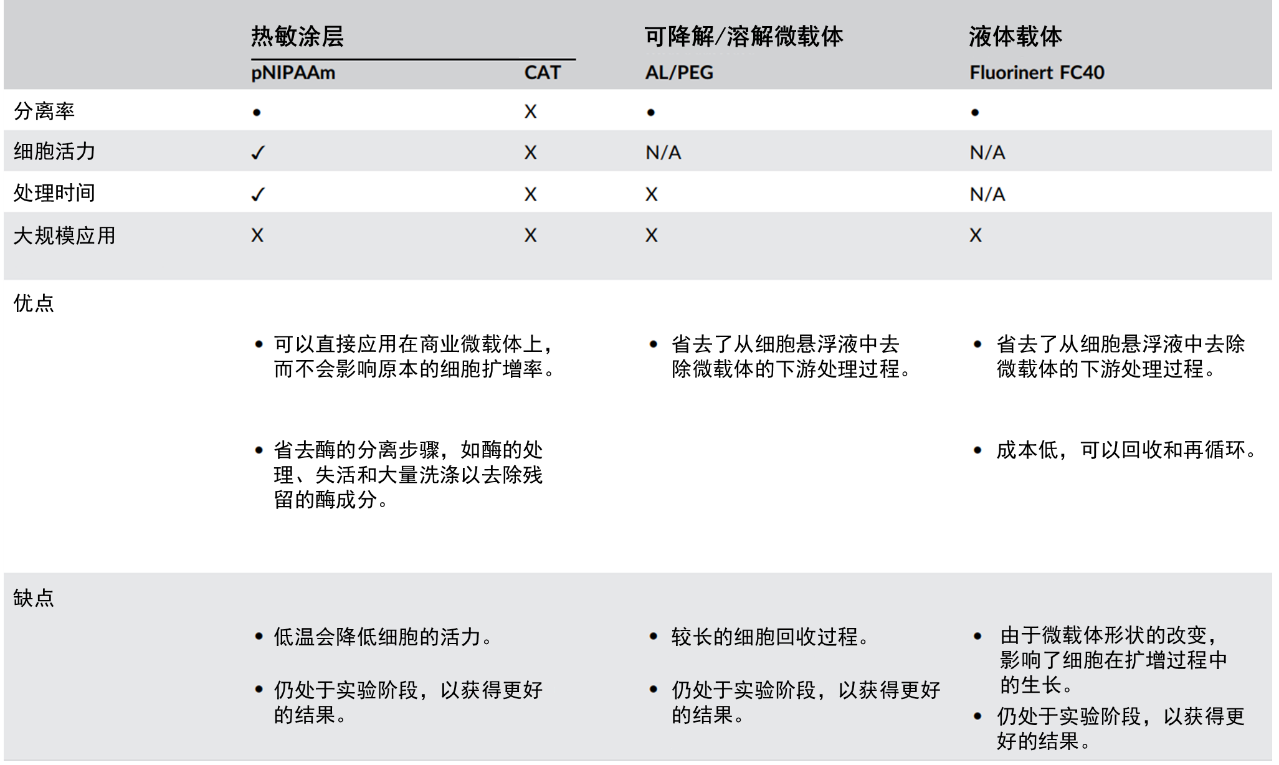
Figure 2: Comparison of different combinations of proteases and microcarriers
To avoid adverse reactions caused by the presence of microcarriers in the human body, high-purity MSC products without microcarriers are required for clinical applications. Therefore, after the harvesting stage, an effective purification process is necessary to separate the cell suspension from the microcarriers. Size exclusion technology is commonly used as the filtration method because the size of microcarriers (ranging from 150 to 250 µm in diameter) is larger than that of MSCs (ranging from 15 to 20 μm in diameter).
However, the filtration technology for MSCs is not yet fully mature. Different filtration methods can be evaluated based on five criteria: the effectiveness of microcarrier filtration, cell recovery rate, cell viability, capability for large-scale filtration, and commercial feasibility of the filtration equipment (Figure 3).
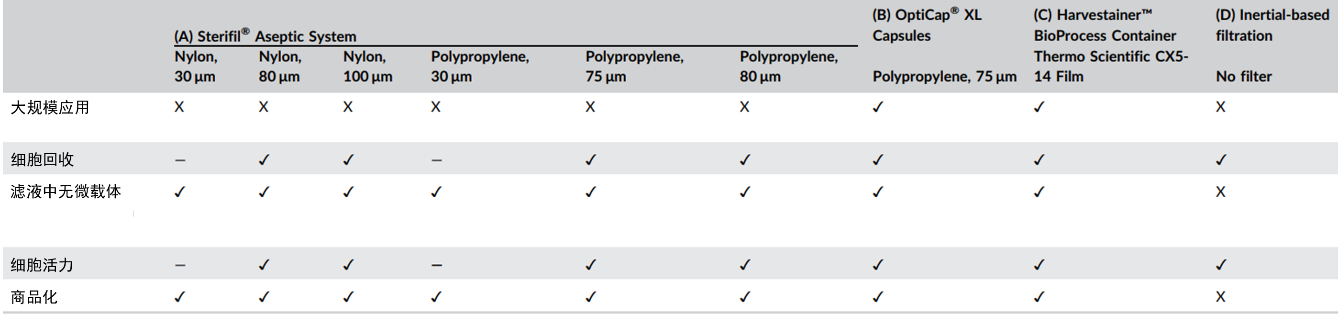
Figure 3: Comparison of Different Filtration Techniques
The final stages of downstream separation process are cell washing and volume concentration, which are typically performed in parallel:
▷ Cell washing is conducted to remove unwanted components such as residual culture media and substrates, enzymes from detachment process, and microcarrier fragments in the cell suspension.
▷ Volume concentration is aimed at meeting the demands of clinical cell therapy, as it requires the delivery of high cell numbers at low doses. For instance, Cunha et al. (Peixoto et al., 2015) estimated a density of 1-8×10^6 cells/mL per kilogram of patient weight, with a total volume of 40 to 50 mL for cell therapy.
The most commonly used method is tangential flow filtration (TFF), and variations of TFF include flat-sheet cassette, polysulfone (PS) and polyethersulfone (PES) techniques. Additionally, continuous centrifugation techniques such as disk-stack centrifuge and fluidized bed can also be utilized.
Similar to the evaluation criteria for filtration techniques, the washing and concentration steps are also assessed based on six indicators: cell recovery rate, cell viability, concentration factor, commercialization feasibility, processing time, and scalability (Figure 4).
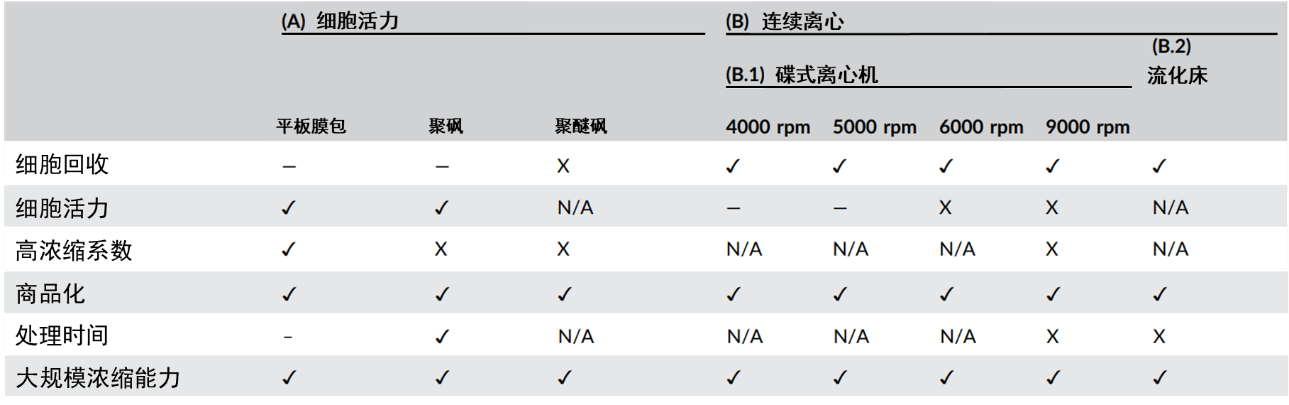
Figure 4:Comparison of Different Concentration Techniques
Finally, the authors have developed a decision tree (Figure 5) that outlines several factors to consider when developing downstream processes and selecting the most appropriate separation technology based on different conditions. By utilizing this information in research, further optimization of the downstream processing of MSCs can be achieved, bringing MSCs as a potential therapeutic approach closer to reality.
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier, is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
