Bio-materials and their combination with cell therapy in the field of medical devices
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-03-28
- Views:36
(Summary description)Bio-materials have good biocompatibility, with less foreign body reaction and increased integration with the host tissue and vascularization compared to synthetic materials. They have the ability to r
Bio-materials and their combination with cell therapy in the field of medical devices
(Summary description)Bio-materials have good biocompatibility, with less foreign body reaction and increased integration with the host tissue and vascularization compared to synthetic materials. They have the ability to r
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-03-28
- Views:36
[Abstract]
Bio-materials have good biocompatibility, with less foreign body reaction and increased integration with the host tissue and vascularization. They can release anti-inflammatory factors, enhance bacterial clearance, and improve healing capacity. The current research goal is to optimize material design to enhance wound healing abilities. Decellularized dermal matrices and collagen are the most commonly used bio-materials. They share the common component of collagen matrix, which has the effects of cell adhesion and proliferation. Therefore, many researchers have developed cellular and decellularized products for various clinical applications, including the treatment of diabetes, chronic diseases, and burn wounds.
[Section 1: Definition of Medical Devices]
Medical devices refer to instruments, equipment, apparatus, in vitro diagnostic reagents and calibrators, materials, and other similar or related items that are directly or indirectly used on the human body, including the necessary computer software. Their effects are mainly obtained through physical means, rather than pharmacology, immunology, or metabolism, or although involving these means, they only play a supporting role.
[Section 2: Definition and Application of Bio-materials]
◉ Bio-materials consist of two parts:
Traditionally derived from human or animal tissues.
The latest biomimetic biodegradable synthetic materials:
These synthetic materials are different from purely synthetic materials as they can degrade or integrate into implanted tissues. Currently, the production of materials includes a range of medical device products, such as decellularized dermal matrices, bone substitutes, and injectables, which are applied in various dermal replacements and burn wounds.
◉ In burn injuries, autologous skin resources are usually limited, and simple skin grafts cannot stably cover the damaged area and restore skin structure and function. There is evidence that skin replacement requires the dermal layer or components that promote dermal layer growth to optimize the healing process and reduce the occurrence of scarring and contracture. Therefore, many experts and scholars use bio-scaffolds combined with cells for various clinical applications, including the treatment of diabetes, chronic diseases, and burn wounds.
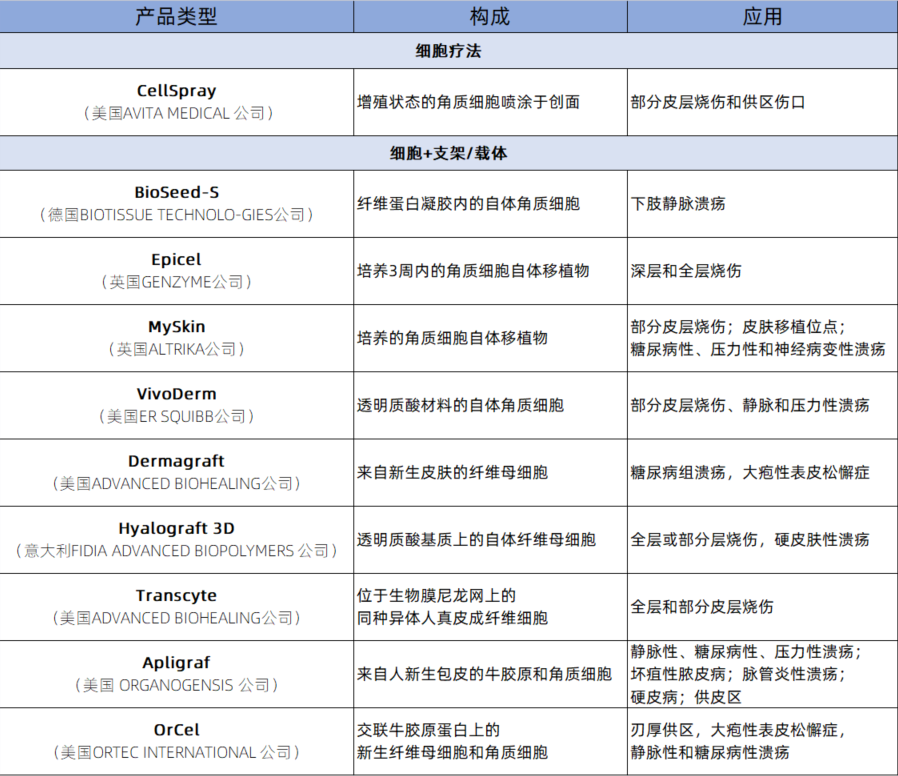
Application of Bio-materials + Cell Therapy in Burn Injuries
Natural materials used to construct natural material scaffolds are derived from cells or tissues, including animal-derived type I collagen and its basement membrane extracts, alginate extracted from seaweed, and chitosan extracted from crab and shrimp shells.
Natural material scaffolds can be classified as follows:
◉ Single-source natural material scaffolds: Made from natural materials derived from cells or tissues (such as collagen, matrix gel, and alginate).
◉ Mixed natural material scaffolds: In addition to natural materials, synthetic materials (such as synthetic basement membranes) are present. The specific applications are shown in the table below:
Injectable gel scaffolds + Wharton's jelly-derived mesenchymal stem cells (WJ-MSCs) in human applications
Functional microgels are easily delivered through minimally invasive injections and seamlessly integrate with surrounding host tissues, making them the preferred carriers for stem cell delivery.
The team led by Dai Jianwu at the Institute of Genetics and Developmental Biology of the Chinese Academy of Sciences has designed an injectable intelligent collagen scaffold material that exists in the form of a hydrogel. They then allow umbilical cord mesenchymal stem cells to attach to this biodegradable collagen scaffold material and inject it into the patient's ovaries, creating a "bed" for stem cell growth and development. By using the scaffold to assist stem cell engraftment and differentiation, the team activates primordial follicles and repairs premature ovarian aging, enabling women with ovarian aging to regain fertility. Additionally, after completing its "mission," the collagen scaffold typically degrades naturally after a few months without causing harm to the human body [1].
Biologically stimulative nanofiber-hydrogel composite materials (NHC) can mimic the microstructure of natural soft tissue matrices, enhancing cell infiltration, immune regulation, and angiogenesis. In a recent study published in "Small," the injection capacity of preformed NHC was improved through mechanical fragmentation, transforming it into microfragmented NHC (mfNHC) in the form of gel particles. This mfNHC can serve as a stem cell carrier, delivering mesenchymal stem cells (MSCs) for soft tissue remodeling [2].
Compared to conventional NHC, mfNHC possesses an equivalent storage capacity but significantly reduces injection resistance. When subcutaneously injected in a rat model, the mfNHC-MSC construct increased the infiltration level of host macrophages compared to using mfNHC alone, leading to greater pro-regenerative polarization, followed by improved vascularization and adipogenesis. This study suggests that using mfNHC as an injectable carrier for cell delivery and soft tissue remodeling holds great potential.
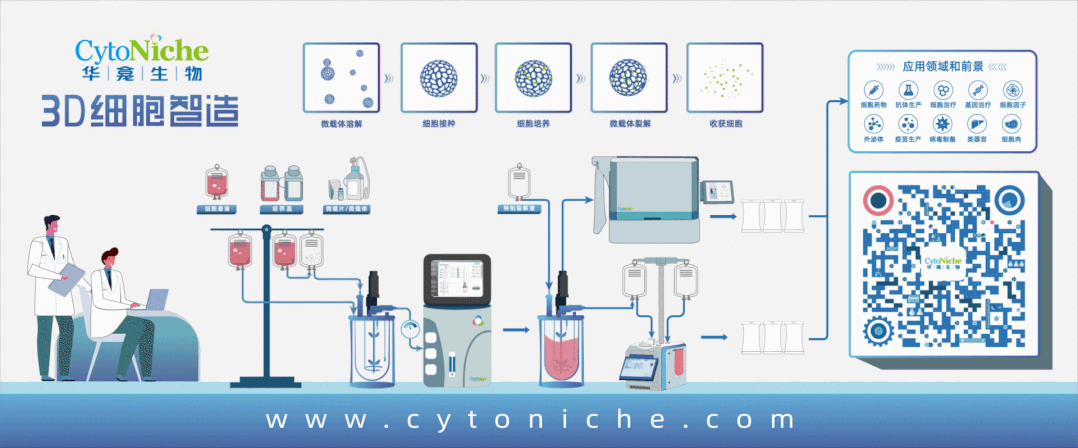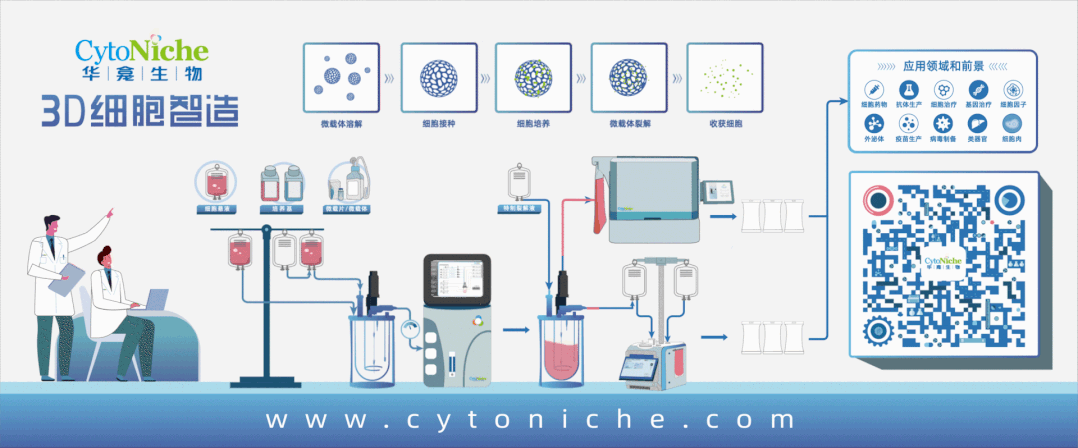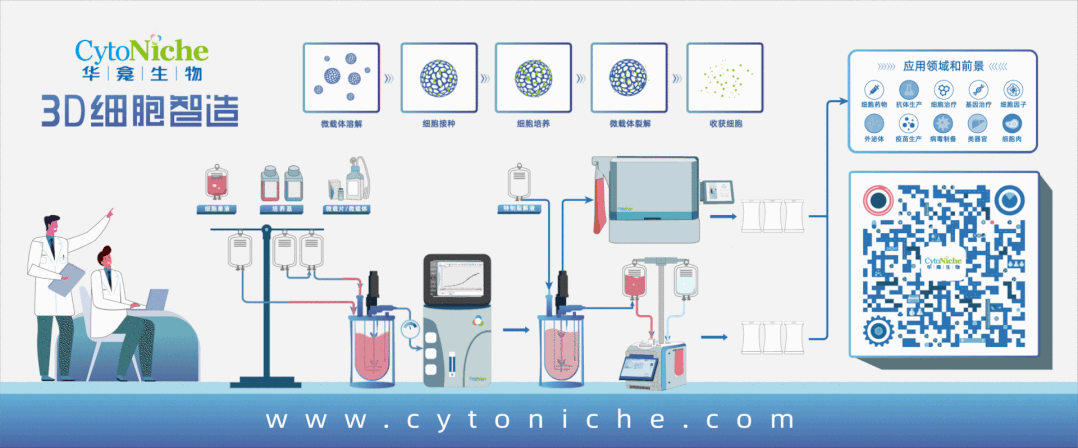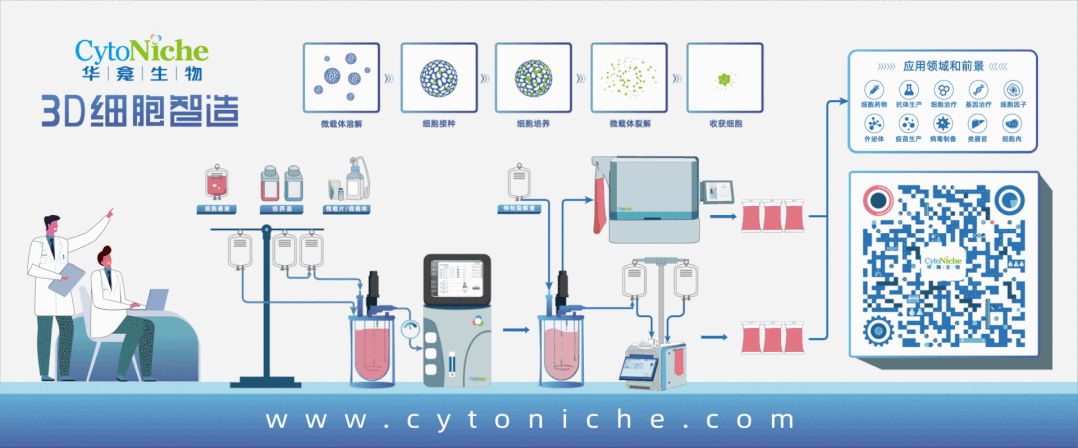
The application of microcarrier scaffold materials in medical devices
Beyond medical aesthetics and orthopedics, the clinical application prospects of tissue regeneration materials combined with living cells are also highly anticipated. Stem cells will become an important development direction for some tissue regeneration material companies. Biodegradable and pharmaceutical-grade microcarriers can be used as scaffold products to prepare more regenerative artificial "living" tissues in combination with stem cells on the basis of composite material scaffolds, bringing better repair and regeneration effects to more severely damaged soft tissues.
3D TableTrix® Microcarriers
The 3D TableTrix® microcarriers have been applied in cell therapy for diseases such as diabetes and osteoarthritis, providing new strategies and methods for the treatment of related diseases.
Click on the image to learn about related research applications.
Click on the image to learn about related research applications.
[References]
[1] Electrospun collagen fibers with spatial patterning of SDF1α for the guidance of neural stem cells. - Adv Healthc Mater. - 2015 - 4(12)
[2] Zhi-Cheng Yao,Biostimulatory Micro-Fragmented Nanofiber-Hydrogel Composite Improves Mesenchymal Stem Cell Delivery and Soft Tissue Remodeling,Small,10 August 2022
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier, is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP





 京公网安备 11010802037749号
京公网安备 11010802037749号
