CytoNiche Biotech's 3D TableTrix® Microcarrier Receives FDA-DMF Type II Qualification Filing Again
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-07-27
- Views:629
(Summary description)Not only Type IV for pharmaceutical excipients, but also Type II for active pharmaceutical ingredients (APIs) and finished drugs!
CytoNiche Biotech's 3D TableTrix® Microcarrier Receives FDA-DMF Type II Qualification Filing Again
(Summary description)Not only Type IV for pharmaceutical excipients, but also Type II for active pharmaceutical ingredients (APIs) and finished drugs!
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-07-27
- Views:629
[Background]
Following the attainment of DMF Type IV and MF Type II qualifications, CytoNiche Biotech's 3D TableTrix® Microcarrier has once again obtained FDA-CDER issued DMF Type II qualification filing, with filing number: 037798.


[About CDER's DMF]
CDER: The Center for Drug Evaluation and Research is responsible for regulating over-the-counter and prescription drugs, including biologics and generics. Its acceptance and review of innovative drugs represent essential information that major pharmaceutical companies globally track and follow in real-time. The associated rigorous evaluations of safety, quality, and effectiveness are widely recognized as the "gold standard."
DMF: Drug Master File encompasses information regarding detailed production facilities, processes, quality controls, materials used in manufacturing, processing, packaging, storing, and distributing human drugs.
I Type: Organization and personnel, facilities and equipment, and standard operating procedures
II Type: Active pharmaceutical ingredients, intermediates, and their raw materials, finished drugs
III Type: Packaging materials
IV Type: Pharmaceutical excipients
V Type: Other information accepted by the FDA
This renewed FDA-DMF Type II qualification filing signifies that CytoNiche Biotech's 3D TableTrix® Microcarrier can be more than just a "silent yet tremendously significant majority" – a pharmaceutical excipient. It can also function as an active pharmaceutical ingredient, becoming the main component in drugs, exerting pharmacological actions or other direct effects on diagnosing, treating, mitigating, preventing diseases, and influencing the structure and function of the human body.
To date, CytoNiche Biotech's 3D TableTrix® Microcarrier has received: ▷ 3 FDA-DMF qualifications: Type II, Type IV, and MF Type II; ▷ 2 China National Medical Products Administration (NMPA) CDE qualifications.
With such comprehensive qualification filings, during product declaration and certification stages, it can provide pharmaceutical companies with authoritative evidence of product quality and safety that's highly suitable for regulatory agencies. It also offers regulatory bodies essential means of supervising and reviewing drugs.
[CytoNiche's Contribution to Declaration]
For numerous pharmaceutical clients, utilizing CytoNiche Biotech's FDA-DMF filing number instead of providing detailed information about materials and excipients during the declaration process can:
▷ Reduce the time required for data preparation, review, and evaluation
▷ Significantly save approval costs and enhance efficiency,
▷ Shorten drug registration cycles and expedite clinical/launch applications.
[CytoNiche's Support Services]
▷ If you are conducting research related to projects using 3D TableTrix® Microcarrier and need to submit applications to the FDA such as Investigational New Drug (IND) or New Drug Application (NDA), you can contact the relevant sales department to apply. CytoNiche Biotech will provide you with an authorization letter that allows the FDA to directly review the technical content of the DMF involved during drug application review, helping to expedite the FDA review process.
▷ If you're interested in CytoNiche Biotech's 3D TableTrix® Microcarrier, you can also scan the code to apply for a trial.

【CytoNiche】
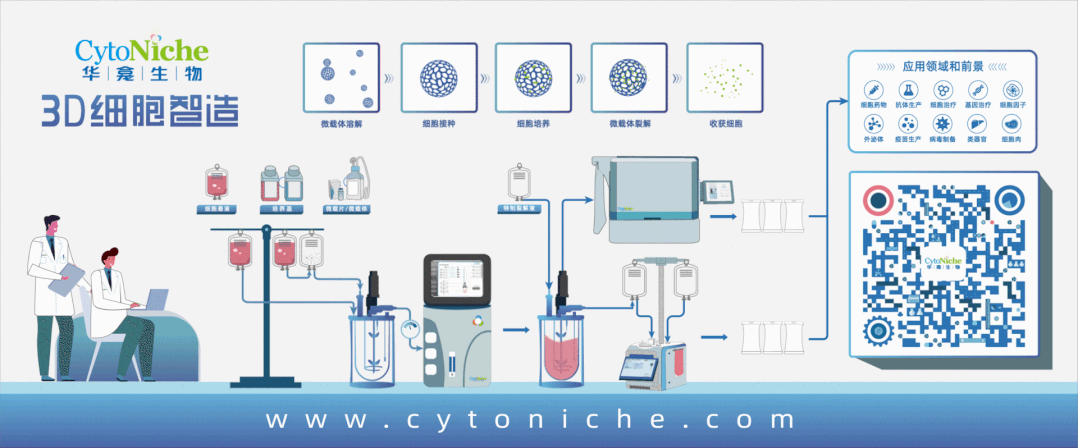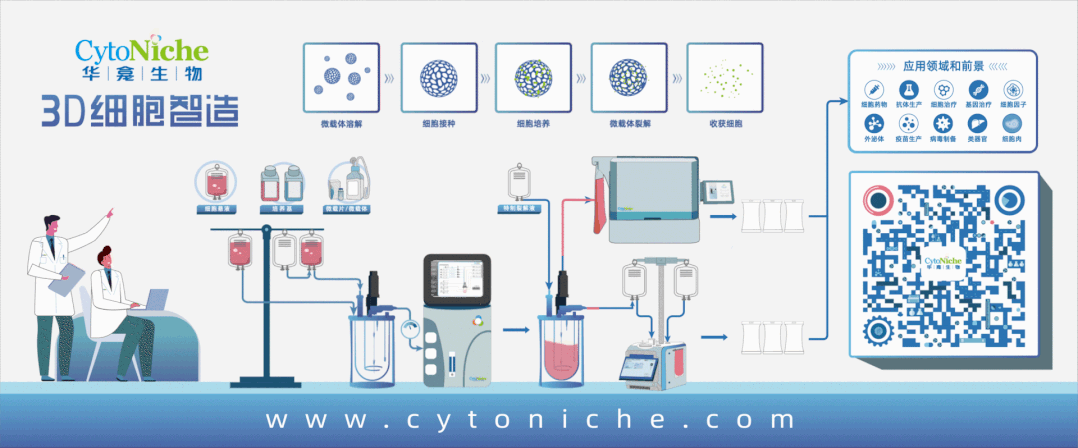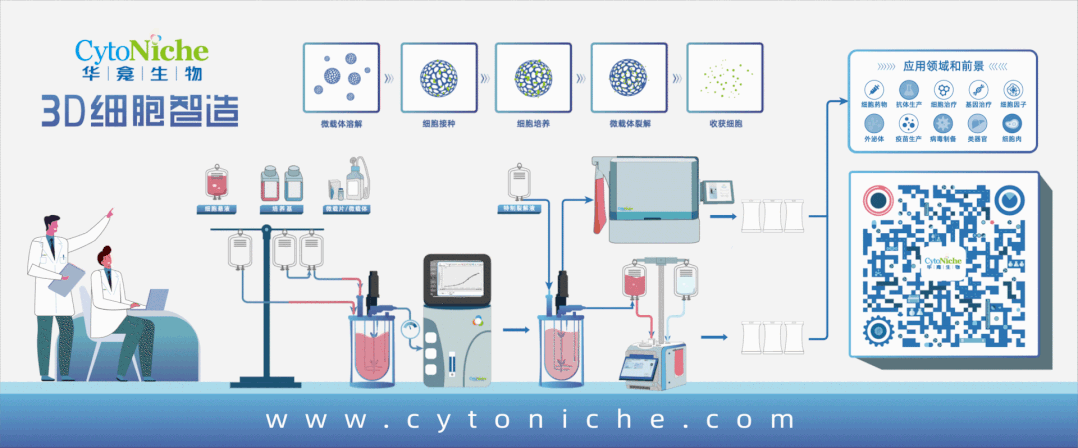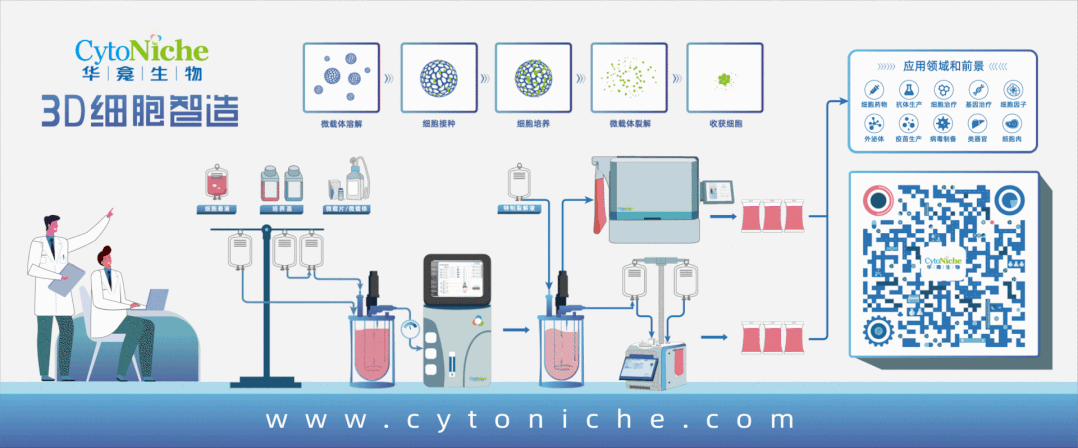
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
