New Product Release | Prelude ②: Application of Stirred Reactor in [Organoid] Culture
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-11-18
- Views:633
(Summary description)"Organoids" technology is an innovative technology of cell culture that uses three-dimensional (3D) culture to form tissue analogs with the spatial structure and partial functions similar to real orga
New Product Release | Prelude ②: Application of Stirred Reactor in [Organoid] Culture
(Summary description)"Organoids" technology is an innovative technology of cell culture that uses three-dimensional (3D) culture to form tissue analogs with the spatial structure and partial functions similar to real orga
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-11-18
- Views:633
["Organoids" technology]
"Organoids" technology is an innovative technology of cell culture that uses three-dimensional (3D) culture to form tissue analogs with the spatial structure and partial functions similar to real organs. Organoid is a key technological breakthrough that has become increasingly popular in recent years, as evidenced by its frequent coverage by many top science journals. The organoid technology has broad application prospects in disease modeling, drug screening, precision medicine, organ development, regenerative medicine, and other fields. Numerous organoids have been created so far, including those of intestinal, kidney, brain, liver, lung, skin, prostate, pancreatic, and retina organoids.
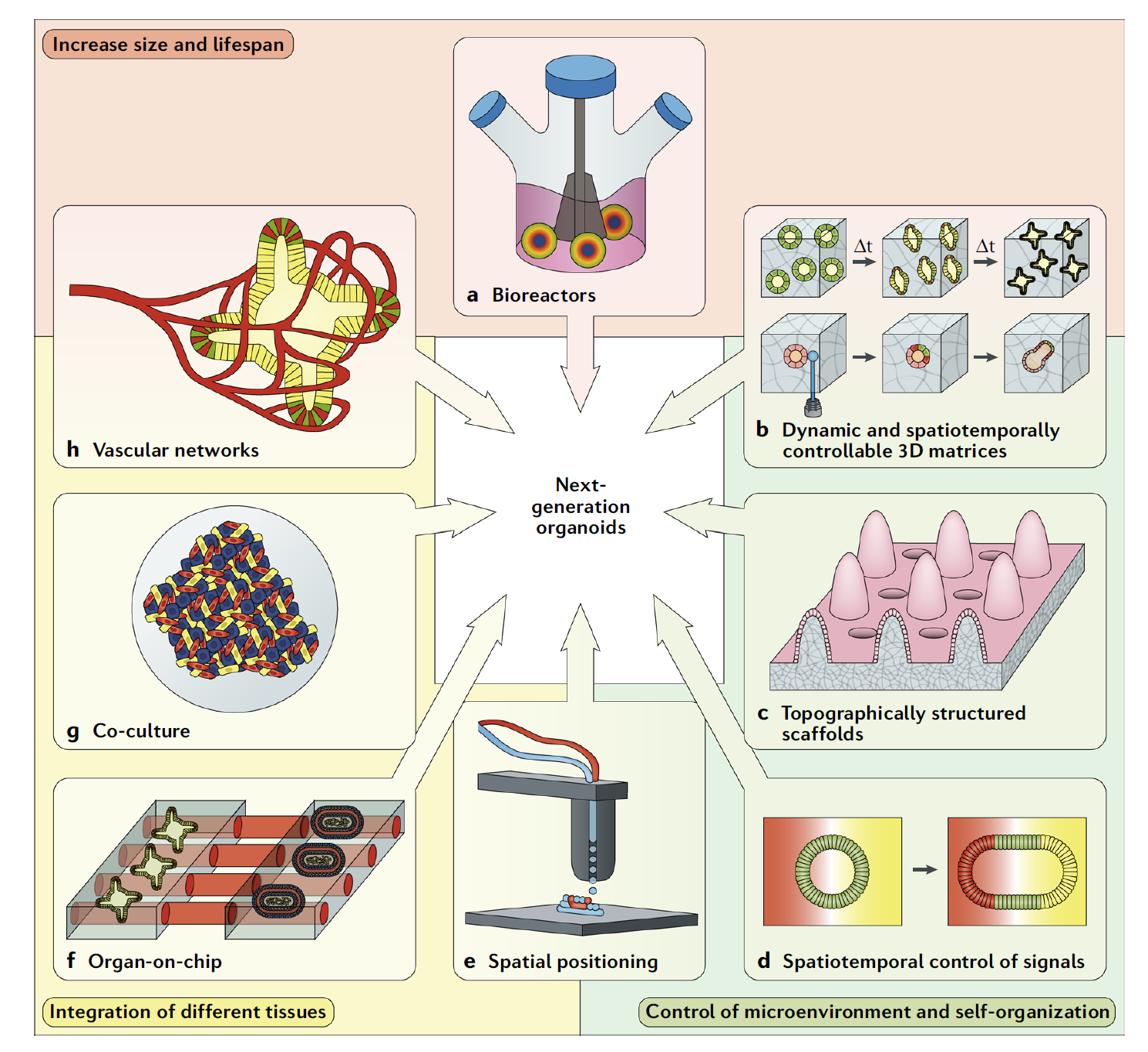
Figure 1: Bioengineering techniques overcome the limitations of existing organoids to create organoids of the next generation [1]
Although the application of organoids is promising, the technology has only been intensively developed in the last decade or so. Many scholars believe that organ maturity of the organoids, consistency, accessibility (i.e., the feasibility of scaled-up production), and clinical-level operational processes are only a few of the many obstacles that organoids must overcome before they can be truly clinically useful. The bioengineering technology is a way for the development of the next generation of organoids to make breakthrough [1-3]. As shown in Figure 1, these engineering tools include bioreactors (a), controlled 3D biomaterials (b), 3D printing (e), organ chips (f), etc.
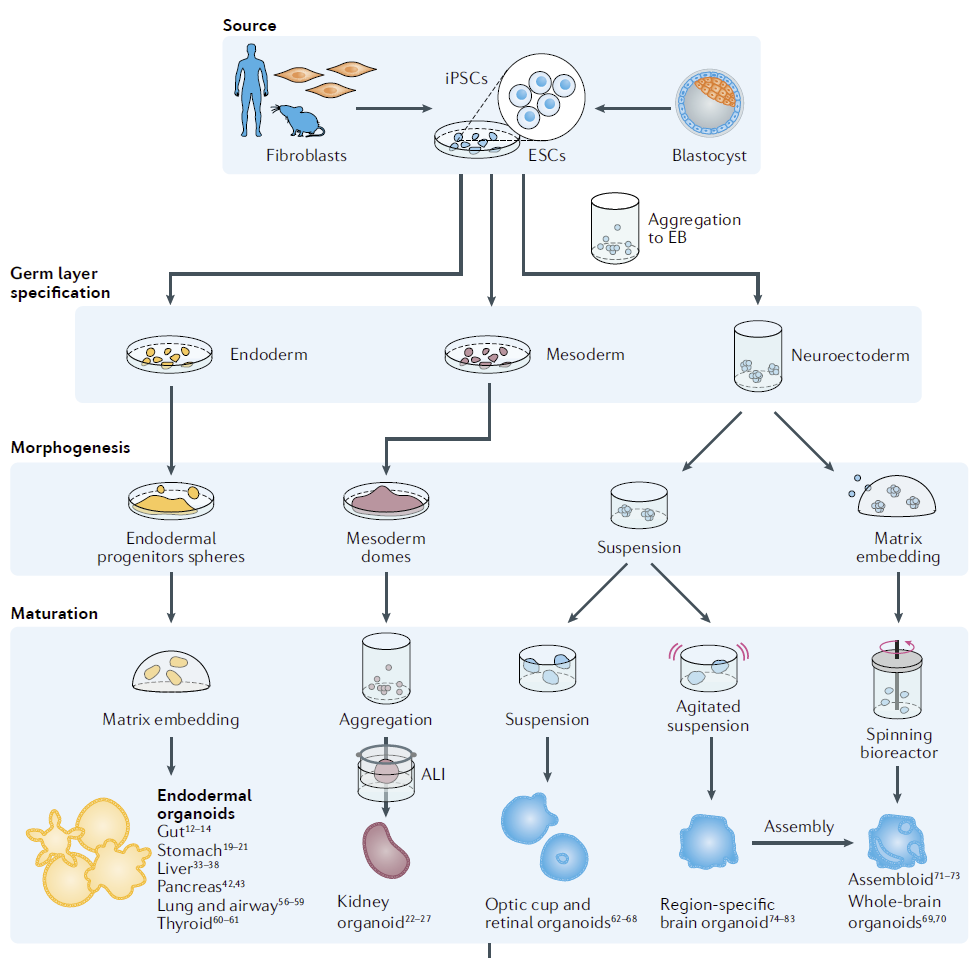
Figure 2: Some of the organoids being cultured in bioreactors for more efficient culture [2].
For organoids derived from pluripotent stem cells (iPSCs), bioreactors are a technological strategy that can overcome the complexity of artificial culture operations, promote organoid maturation, and improve the capacity and homogeneity of organoid preparation [2-4]. Previously, stirred bioreactors were utilized in the creation of iPSC embryoids and the differentiation of three germ layers [3, 4]. But in recent years, stirred bioreactors have been used to promote the maturation of different organoids (Figure 2) [2].
[Results of relevant studies]
Studies have demonstrated that the structural complexity and functional maturity of an organoid are proportional to its size, with larger organoids capable of creating more complex structures, typically ranging in size from 4 mm to 200 mm. Naturally, the larger the organoid, the more difficult it is to provide sufficient nutrients and oxygen to the cells inside the organoid. This is where dynamic culture systems, such as bioreactors, prove their values. They can provide adequate nutrient and oxygen exchange to induce the maturation and maintenance of large volume organoids [4]. Several studies on the use of stirred bioreactors for the preparation of organoids are listed below.
● Brain organoids
Lancaster's trailblazing study published in Nature in 2013 reported brain organoids with co-existing but clearly distinct brain regions. This was the world's first ever project where brain organoids were used to establish a human case model [5]. Pluripotent stem cells were first cultured in EB form and then induced to differentiate into neuroepithelial tissues for culture. After that, the neuroepithelial tissues were embedded in Matrigel and transferred to a stirred reactor for culture after 4 days of static culture. In this way, 4-mm-sized organoids that faithfully replicate brain structures can be obtained.
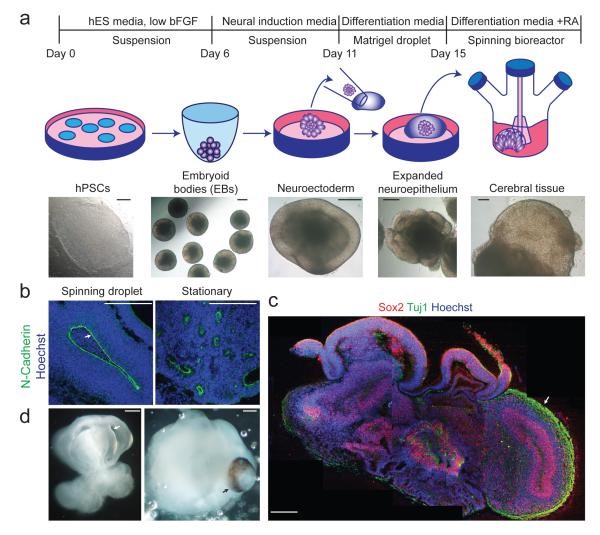
Figure 3: Culture of brain organoids in a stirred reactor [5]
Qian et al. published a paper in Cell in 2016, introducing the use of micro-stirred bioreactors, through the addition of different induction and differentiation media, to produce organoids in a single brain region, such as the forebrain region and midbrain region, hippocampus organoids. The so produced organoids were used for studying Zika virus infection [6]. The team later published an article in Nature Protocols to guide how to culture brain organoids using micro-bioreactors. The article points out that micro-reactors are capable of achieving a culture time of over 200 days.
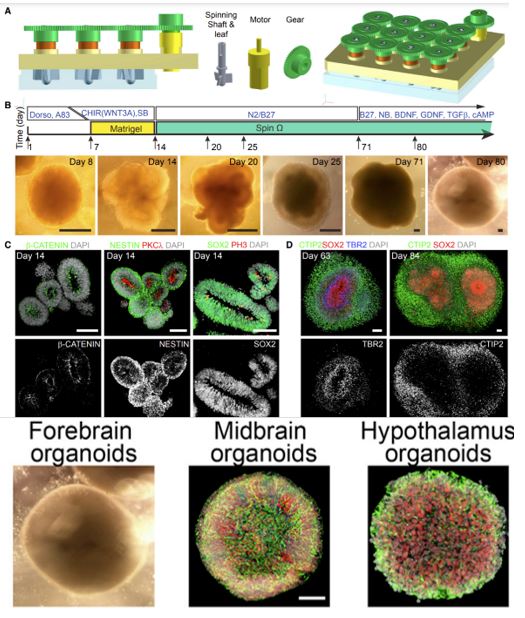
Figure 4: Micro-reactors for the formation of forebrain, midbrain, hindbrain, and hippocampus organoids [6]
More recent studies on brain organoids also include the article published in Nature by Velasco in 2019, which also described the use of stirred bioreactors to construct brain organoids. Using single-cell sequencing technology, the researchers found that after three to six months of development, the cell diversity of brain organoids rose dramatically and resembled the cell composition of the human embryonic cerebral cortex. They also discovered that the formation of cell types between individual brain organoids was precisely reproducible.
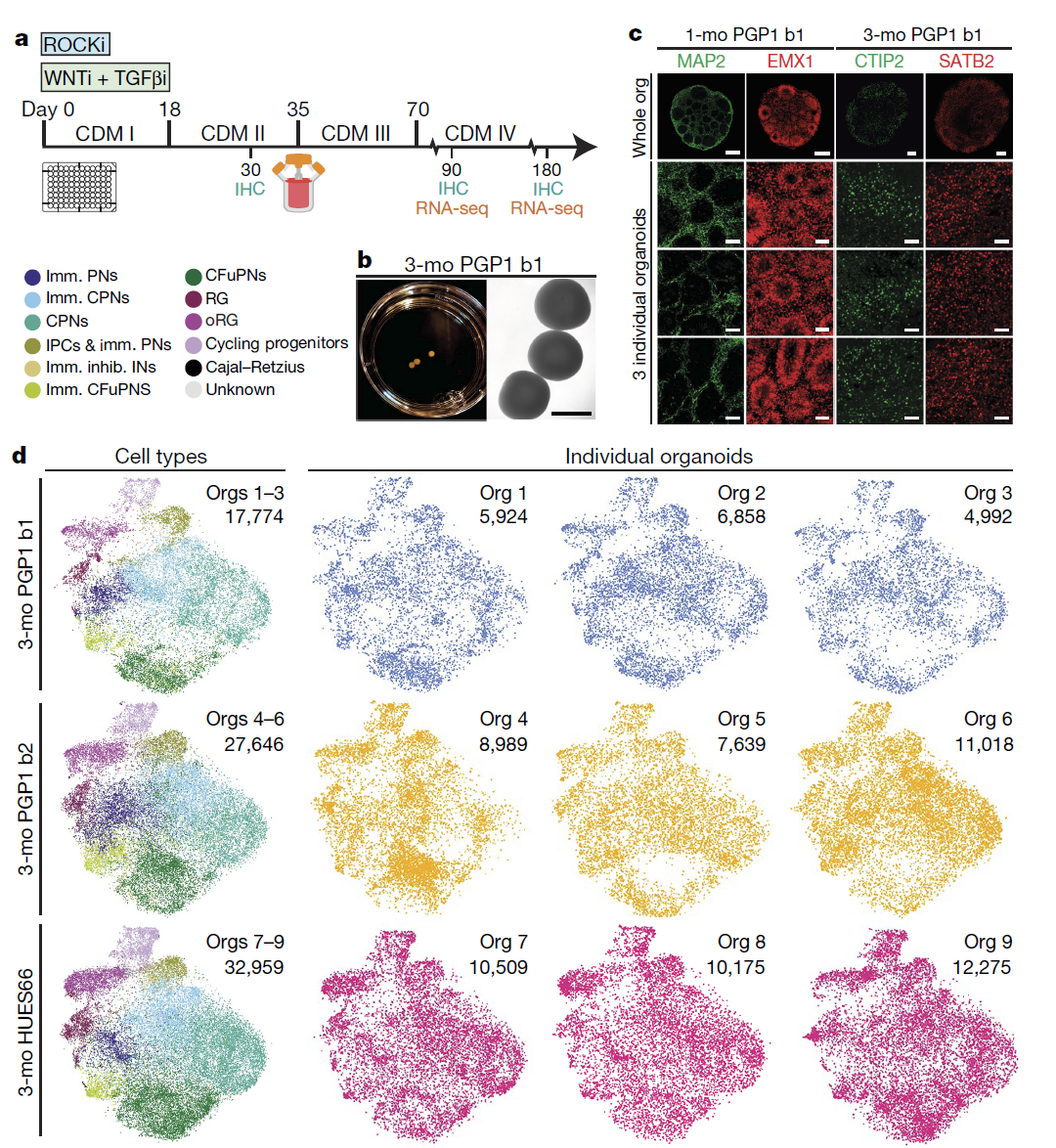
Figure 5. Stirred bioreactor-formed brain organoids are similar to the diversity of human cerebral cortex cells and stable reproducibility is achieved between individual organoids [7]
● Retinal organoids
The classical retinal organoid culture method was published in Nature by Eiraku et al. in 2011. Since then, the culture method for retinal organoids has essentially followed the suspension culture approach. In 2018, Ovando-Roche et al. released a study on the utilization of stirred reactors for the development of retinal organoids. Their experiments showed that the use of bioreactors improved the laminar layered structure of retinal organoids and increased the production of photoreceptor cells with cilia and nascent outer segment-like structures [8]. The use of bioreactor culture was also found to decrease the apoptosis of organoids and increase cell proliferation. They indicated that the stirred reactor was expected to accelerate the culture of retinal cells for practical clinical application.
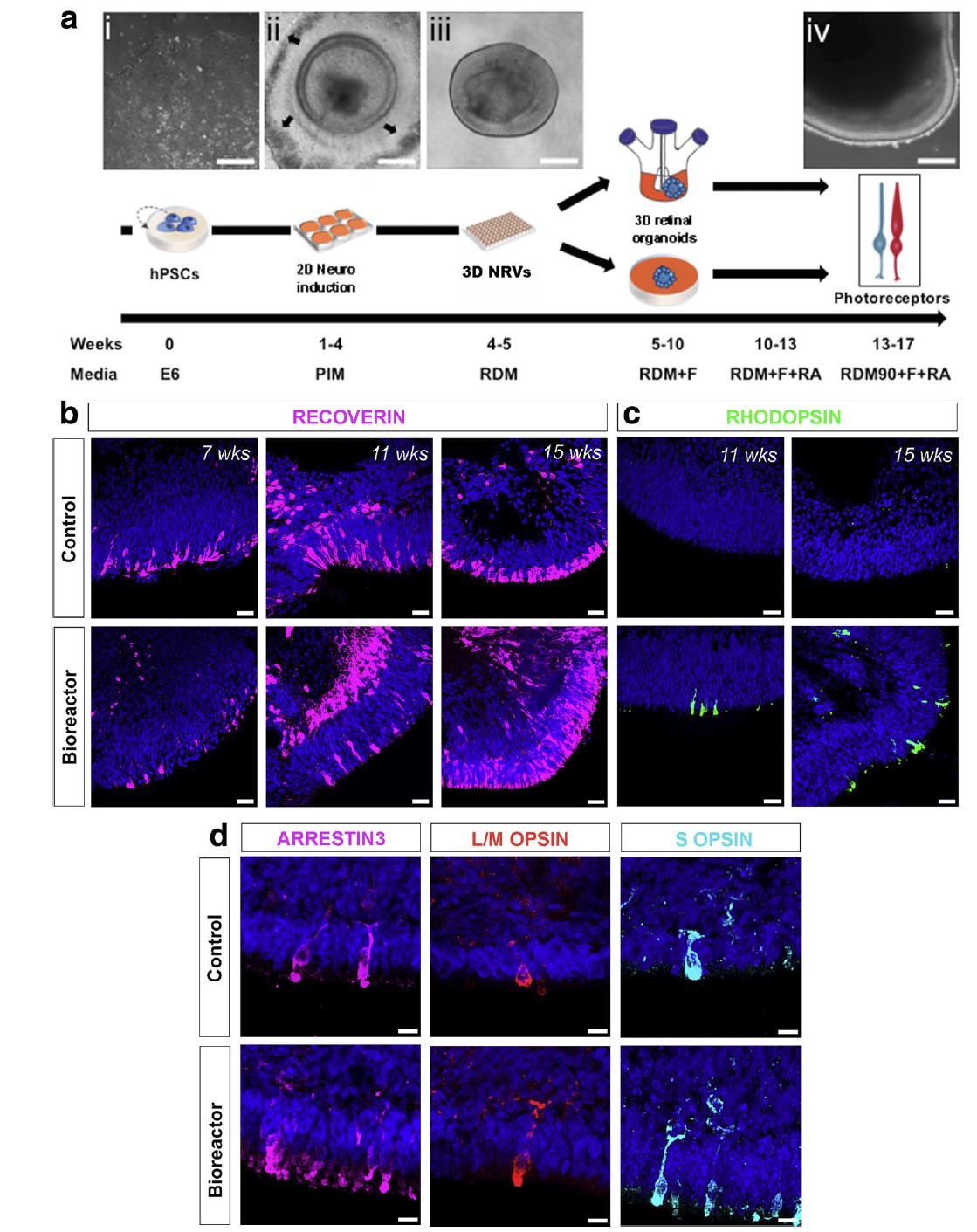
Figure 6. Stirred bioreactors promote the generation of retinal organoids [8]
● Liver organoids
In the same year when Lancaster published the article about brain organoids, Baharvand's research group published research results on the use of stirred bioreactors to induce the differentiation of pluripotent stem cells into hepatic-like cells [9]. They went on to publish in 2020 research results on liver organoids, in which they discovered that dissolved oxygen content in the culture system can regulate the differentiation of pluripotent stem cells into liver organoids [10]. By controlling the dissolved concentration from 20% to 40%, it is possible to promote the differentiation of pluripotent stem cells into endoderm, and by further controlling oxygen concentration at 30%, it is possible for pluripotent stem cells to achieve efficient differentiation into liver organoids containing red blood cells and functional hepatocytes.
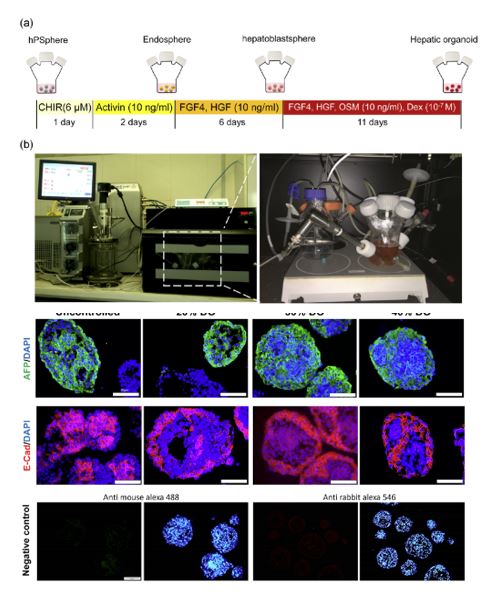
Figure 7. The formation of liver organoids can be regulated by controlling the amount of oxygen dissolved in a stirred reactor [10]
● Kidney organoids
In a study published in Stem Cell Reports in 2018, Przepiorski et al. adopted a simple, inexpensive, and user-friendly approach for culturing kidney organoids [11]. In this technique, pluripotent stem cells were cultured in the form of embryoid for 8 days to form a tubular structure and then transferred to a 125 mL stirred reactor for culture up to 26 days. The optimal tissue morphology took shape as early as on Day 14. By comparing with fetal human kidney, the data showed that the organoid tissue on Day 14 was most similar to the late capillary loop nephron. This research protocol provides a rapid, efficient, and cost-effective method for generating large quantities of human fetal kidney tissue.
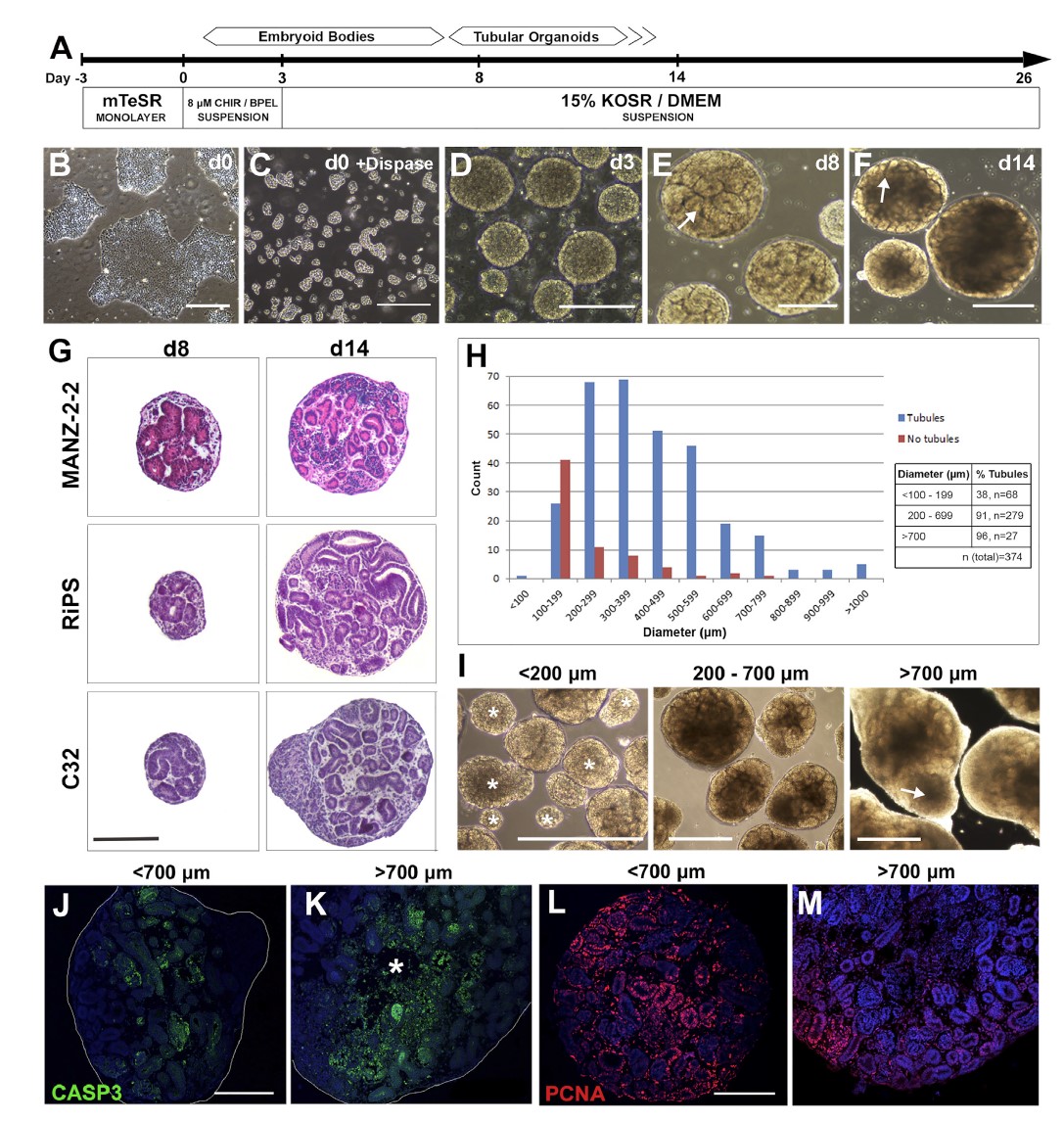
Figure 8. A simple method for culturing embryoid bodies of pluripotent stem cells and kidney organoids in suspension culture in a stirred reactor [11]
[Summary]
Stirred bioreactors have shown broad application prospects in some types of organoid and are helping with the advancing of the organoid technology. More researchers in this emerging field are looking to explore, learn from, and integrate with known biotechnologies to advance the transformation and application of organoids. However, more efforts are needed to broaden the application of stirred bioreactors to more organoid types.
Due to the difficulties and expense of organoid culturing and the enormous size of typical stirred reactors, it is challenging to use them extensively in early-stage research. Micro stirred reactors like those created by Qian et al. will be an innovative research tool that encourages more scientists to investigate the combination of bioengineering and organoid technologies. The reactor developed by Qian et al. has not been officially put into the market, and although the system is desirably small, it cannot be automatically controlled, and a single reactor can be used for verifying one set of experimental conditions only.
To address the pain points in organoid research, such as large investment, long test period, and complex culture condition screening, as well as the applicability of existing stirred reactors in different fields and stages, a 3D FloTrix® 6-channel micro bioreactor will be rolled out by CytoNiche Biotechnology Co. Ltd., for the purpose of cell drug research and development, organoid culture, virus packaging, plasmid transfection, gene and protein expression, tumor research and many other fields, providing efficient, stable and economical innovative culture methods to accelerate the industrialization of biopharmaceuticals. Coming soon on November 11!
References
[1] Rossi, G., Manfrin, A., & Lutolf, M. P. (2018). Progress and potential in organoid research. Nature Reviews Genetics. doi:10.1038/s41576-018-0051-9
[2] Hofer, M., Lutolf, M.P. Engineering organoids. Nat Rev Mater 6, 402–420 (2021).
https://doi.org/10.1038/s41578-021-00279-y
[3] Yin, X., Mead, B. E., Safaee, H., Langer, R., Karp, J. M., & Levy, O. (2016). Engineering stem cell organoids. Cell stem cell, 18(1), 25-38.
[4] Silva, T. P., Cotovio, J. P., Bekman, E., Carmo-Fonseca, M., Cabral, J., & Fernandes, T. G. (2019). Design principles for pluripotent stem cell-derived organoid engineering. Stem Cells International, 2019.
[5] Lancaster, M. A., Renner, M., Martin, C. A., Wenzel, D., Bicknell, L. S., Hurles, M. E., ... & Knoblich, J. A. (2013). Cerebral organoids model human brain development and microcephaly. Nature, 501(7467), 373-379.
[6] Qian, X., Nguyen, H. N., Song, M. M., Hadiono, C., Ogden, S. C., Hammack, C., ... & Ming, G. L. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell, 165(5), 1238-1254.
[7] Velasco S, Kedaigle A J, Simmons S K, et al. Individual brain organoids reproducibly form cell diversity of the human cerebral cortex[J]. Nature, 2019, 570(7762): 523-527.
[8] Ovando-Roche, P., West, E. L., Branch, M. J., Sampson, R. D., Fernando, M., Munro, P., ... & Ali, R. R. (2018). Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem cell research & therapy, 9(1), 1-14.
[9] Vosough, M., Omidinia, E., Kadivar, M., Shokrgozar, M. A., Pournasr, B., Aghdami, N., & Baharvand, H. (2013). Generation of functional hepatocyte-like cells from human pluripotent stem cells in a scalable suspension culture. Stem cells and development, 22(20), 2693-2705.
[10] Farzaneh, Z., Abbasalizadeh, S., Asghari‐Vostikolaee, M. H., Alikhani, M., Cabral, J. M., & Baharvand, H. (2020). Dissolved oxygen concentration regulates human hepatic organoid formation from pluripotent stem cells in a fully controlled bioreactor. Biotechnology and Bioengineering, 117(12), 3739-3756.
[11] Przepiorski, A., Sander, V., Tran, T., Hollywood, J. A., Sorrenson, B., Shih, J. H., ... & Davidson, A. J. (2018). A simple bioreactor-based method to generate kidney organoids from pluripotent stem cells. Stem Cell Reports, 11(2), 470-484.
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
