Delegation Reception | Tianjin Customs Visited Tianjin CytoNiche for Inspection and Research
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-10-26
- Views:841
(Summary description)Recently,theteamofProfessorDuYananofTsinghuaUniversitySchoolofMedicine,togetherwiththeteamofDirectorMengShufangoftheChinaInstituteofFoodandDrugControl,theteamofWangBinofNanjingDrumTowerHospital,andBei
Delegation Reception | Tianjin Customs Visited Tianjin CytoNiche for Inspection and Research
(Summary description)Recently,theteamofProfessorDuYananofTsinghuaUniversitySchoolofMedicine,togetherwiththeteamofDirectorMengShufangoftheChinaInstituteofFoodandDrugControl,theteamofWangBinofNanjingDrumTowerHospital,andBei
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-10-26
- Views:841
[Arrival]
On October 21, 2022, Liu Xintong, deputy director of Tianjin Customs, Li Lun, chief of Tianjin Customs Statistics Department, Wang Yuan, director of Wuqing Customs, and Zhang Liang, deputy director of Wuqing Customs, visited Tianjin CytoNiche Co., Ltd. (hereinafter referred to as Tianjin CytoNiche) for inspection. Tianjin CytoNiche General Manager Tao Hongchen, Quality Director Liu Limeng, Administrative Director Sun Baoming and others received the Tianjin Customs delegation.

[Visit and Inspection]
Director Liu Xintong first visited the production workshop and laboratory of Tianjin CytoNiche. During this period, Mr. Liu Limeng took the delegation through the company's main products, manufacturing process, layout of production and testing areas, etc.


[Discussion and Exchanges]
At the subsequent meeting, general manager Tao Hongchen made a detailed report on the company's operation performance, research and development achievements, future planning and export. Director Liu Xintong listened carefully to the report and had an in-depth understanding of the main content.

[Support and Guidance]
Learning that CytoNiche commits itself to promoting domestic technology and products, attaches great importance to overseas markets, and intends to increase exports in the future, Liu Xintong, deputy director of Tianjin Customs, praised CytoNiche for its efforts, and said that Tianjin Customs would try its best to meet the needs for the company's development, and help expedite product inspection and testing, shorten customs clearance time, avoid packaging damage, and protect intellectual property rights on the basis that all instructions of the General Administration of Customs are followed.
Deputy Director Liu Xintong also gave valuable suggestions for CytoNiche’s future customs declaration and clearance as well as customs-related work. He pointed out that due to the originality of the company's products, the company should make sure that the ways to classify similar products at home and abroad should be kept consistent and that close attention should be paid to preventing and combating potential intellectual property infringements. In the future customs declaration for export, Tianjin Customs will work with the company to make adequate preparation and always maintain a dual communication mechanism to ensure that the company can complete its future customs clearance for export products efficiently and smoothly.

【CytoNiche】
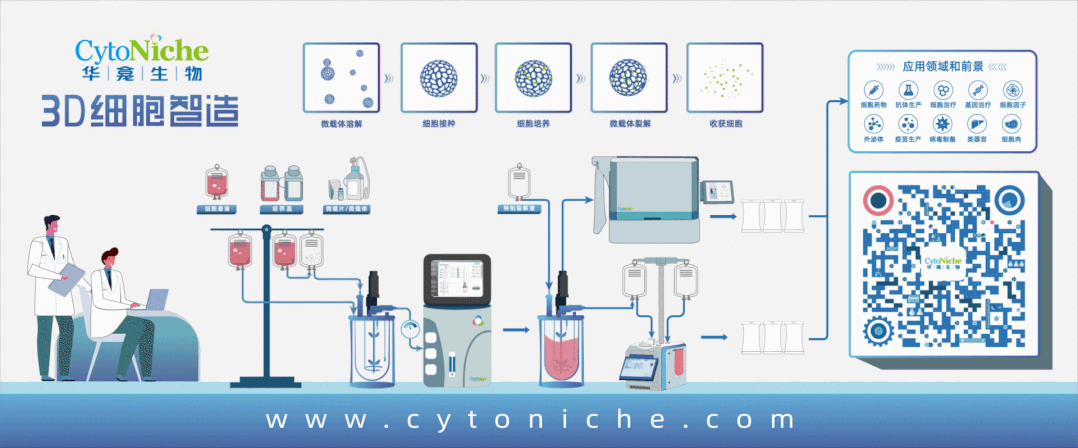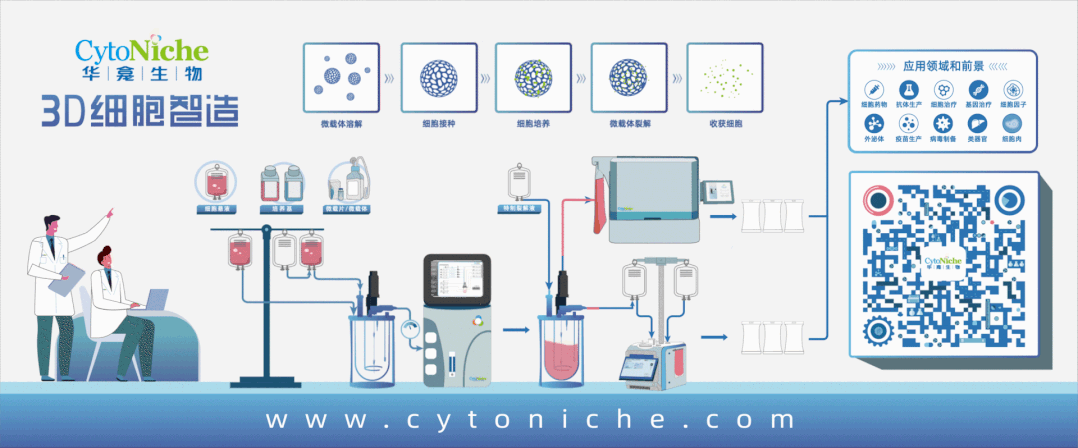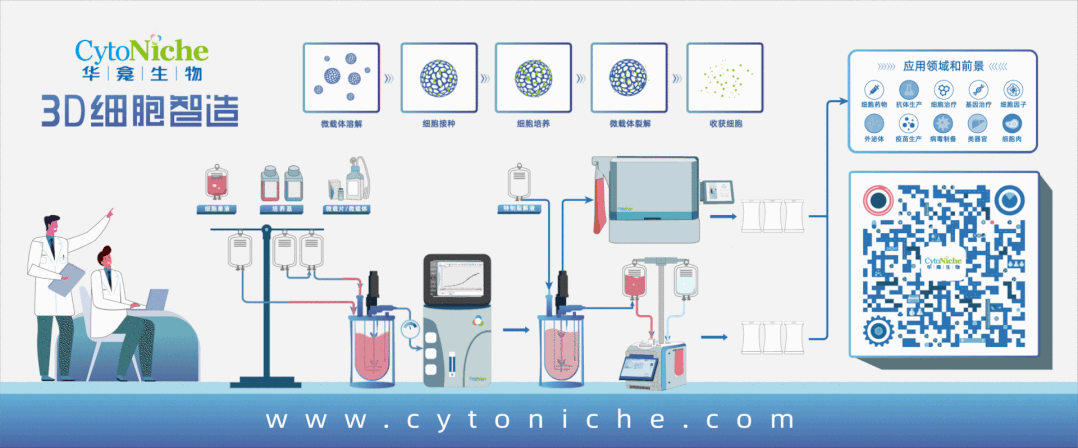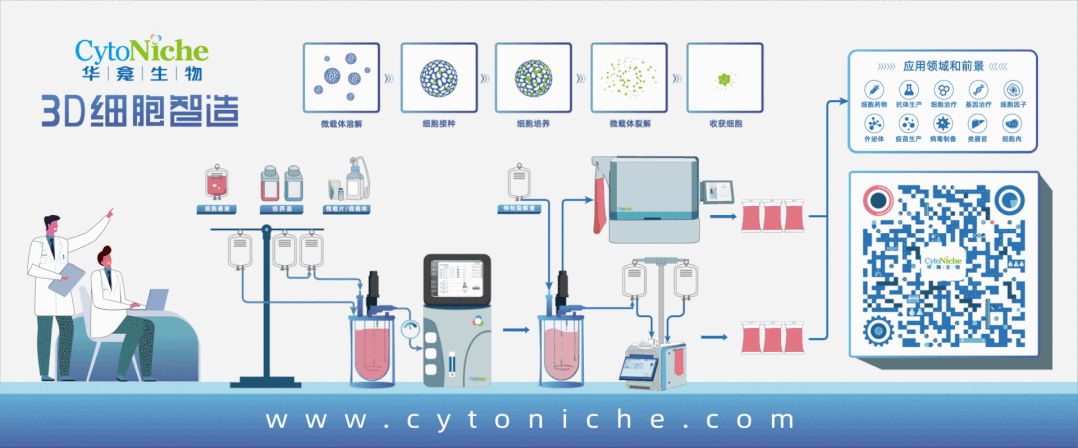
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
