Production Protocol for Single-Batch 10 Billion Stem Cells [Automated Closed Industrial Scale Cell Production (ACISCP) based on GMP-grade 3D microcarriers] Published Online by Tsinghua University & National Institutes for Food and Drug Control & CytoNiche
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-09-22
- Views:841
(Summary description)Recently,theteamofProfessorDuYananofTsinghuaUniversitySchoolofMedicine,togetherwiththeteamofDirectorMengShufangoftheChinaInstituteofFoodandDrugControl,theteamofWangBinofNanjingDrumTowerHospital,andBei
Production Protocol for Single-Batch 10 Billion Stem Cells [Automated Closed Industrial Scale Cell Production (ACISCP) based on GMP-grade 3D microcarriers] Published Online by Tsinghua University & National Institutes for Food and Drug Control & CytoNiche
(Summary description)Recently,theteamofProfessorDuYananofTsinghuaUniversitySchoolofMedicine,togetherwiththeteamofDirectorMengShufangoftheChinaInstituteofFoodandDrugControl,theteamofWangBinofNanjingDrumTowerHospital,andBei
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-09-22
- Views:841

Recently, the team of Professor Du Yanan of Tsinghua University School of Medicine, together with the team of Director Meng Shufang of the China Institute of Food and Drug Control, the team of Wang Bin of Nanjing Drum Tower Hospital, and Beijing Huagong Biotechnology Co., Ltd. and Beijing Beilai Biotechnology Co., Ltd. jointly published the title "GMP‐grade" in the Journal of Tissue Engineering and Regenerative Medicine Microcarrier and automated closed industrial scale cell production platform for culture of MSCs" research paper.
The study found that the 3D FloTrix® process based on GMP-grade 3D microcarrier (including step-by-step scale-up bioreactor and cell automatic harvesting device) can realize the production of automated closed-ended industrial-grade stem cells, and the number of cells can reach 10 billion in 13 days, and more than 20 billion cells can be obtained and prepared in a single batch using the 3D FloTrix® vivaPREP. The whole production process adopts a fully enclosed automated process to detect the number and quality of cells in real time, and finally the cell preparation implements strict quality inspection and library standards, and successfully realizes the production of high-quality industrial-grade stem cell preparations.
【Research Background】
Mesenchymal stem/stromal cell, the industrialization of MSC is one of the great challenges of current clinical applications of cell therapy and regenerative medicine. At present, the MSC industrial production process mainly uses the cell factory for two-dimensional (2D) plane culture (Figure 1), which relies on labor, so it will lead to high labor costs, easy pollution, the production process can not detect cell quality in real time, low single-batch yield, poor product stability between batches and other issues.
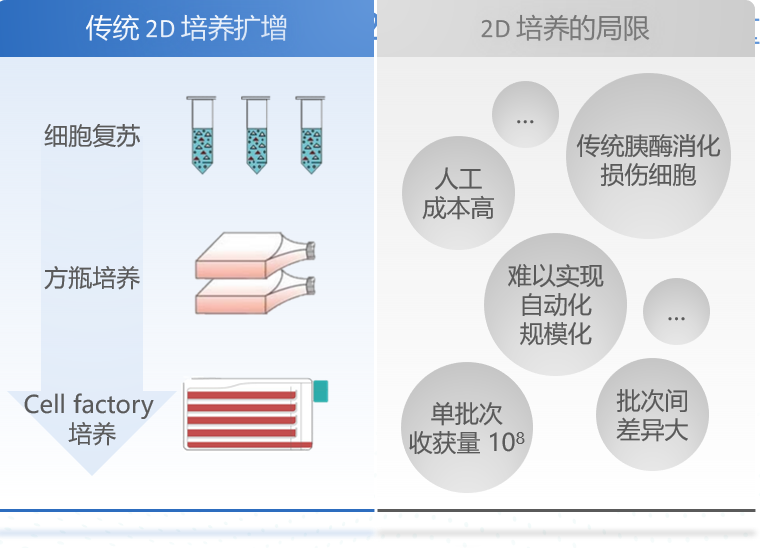
Figure 1: Traditional Stem Cell Manufacturing Process and Limitations
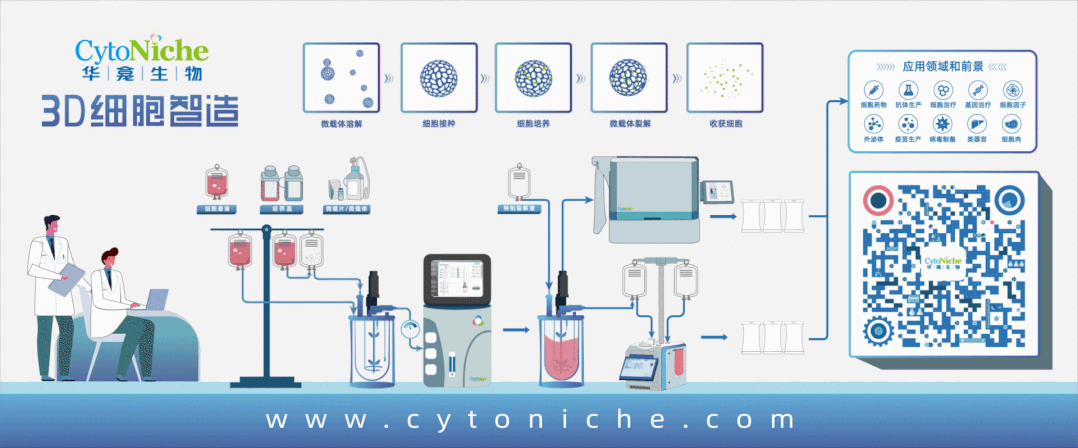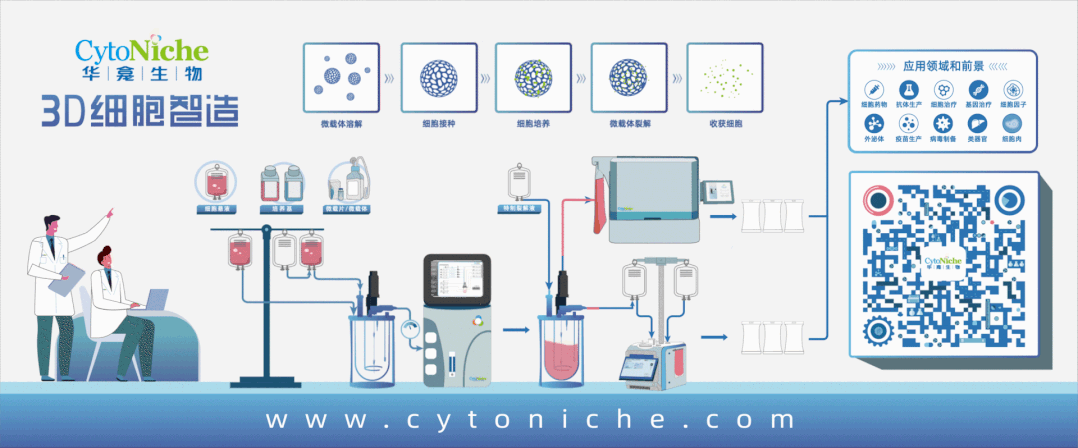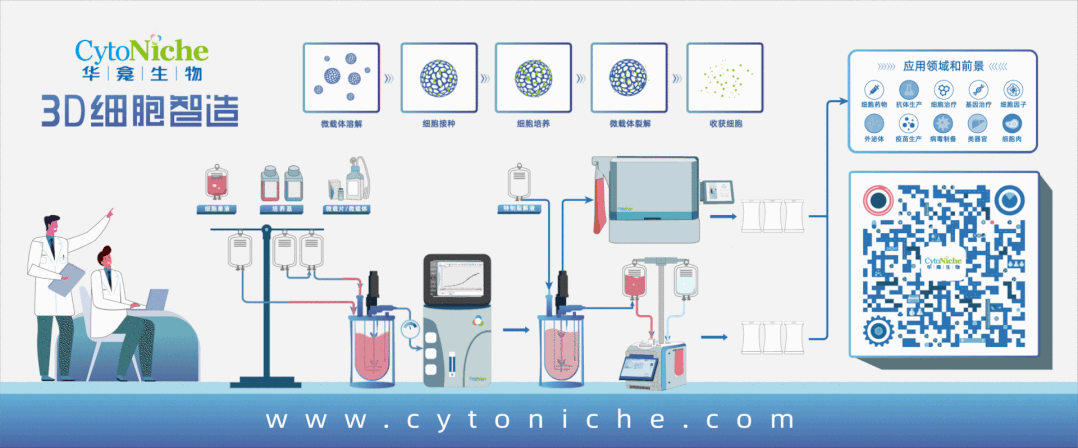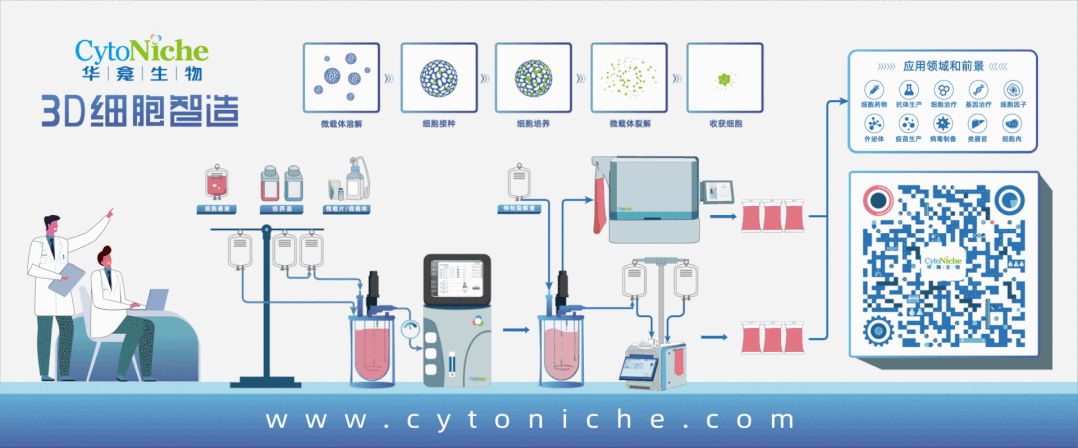
In order to overcome the shortcomings of 2D culture and facilitate the industrialization development of stem cell therapy, Du Yanan's research group at Tsinghua University, in conjunction with Director Meng Shufang of the National Institutes for Food and Drug Control and Director Wang Bin of Nanjing Drum Tower Hospital, jointly created the automated closed industrial scale stem cell production (ACISCP) platform based on GMP-grade 3D microcarriers. A three-grade 3D FloTrix ® vivaSPIN bioreactor is utilized via this platform to complete industrial-grade cell amplification in 13 days, and more than 20 billion cell preparations are obtained in a single batch using the 3D FloTrix® vivaPREP automated cell harvesting device. An automated fully-closed process is utilized in ACISCP process to detect the quantity and quality of cells in real time, and the criteria of the quality control and library construction are strictly followed for final cell preparations to successfully achieve the production of high-quality industrial grade stem cell preparations (Figure 2) and facilitate the rapid development of the cell therapy industry.
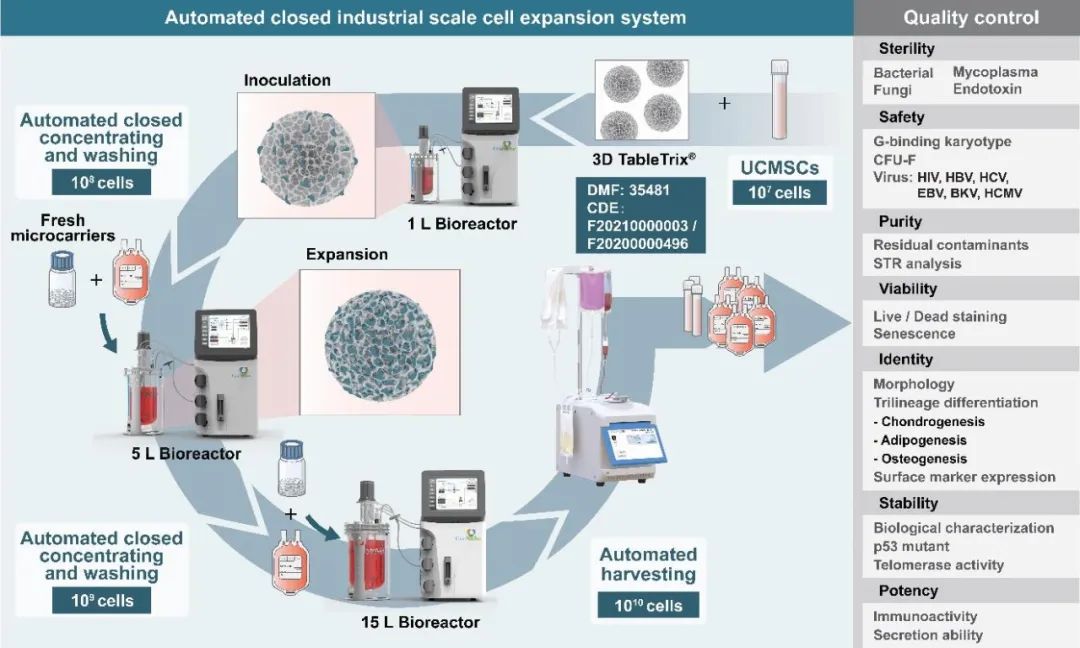
Figure 2: Production Schematic of Automated Closed Industrial-grade Stem Cells
【Conclusion】
① Characteristics of GMP-grade 3D TableTrix® Microcarrier
3D TableTrix® microcarriers are tablets made from 100-200 μm of microcarriers (Figure 3-A) and disperse once upon contact with water. Electron microscopy displays that 3D microcarriers exhibit porous structures, and mesenchymal stem cells can attach to the interior of the carriers (Figure 3-B) and grow in a three-dimensional environment. Cell harvesting is accomplished by degrading the microcarrier with the special microcarrier lysis 3D FloTrix Digest® (Figure 3-C). Released cells are harvested without damage. In this article, 3D TableTrix® microcarriers are used to achieve efficient amplification of umbilical cord mesenchymal stem cells (UCMSC), adipose-derived mesenchymal stem cells (ADMSC), and dental pulp mesenchymal stem cells (DPSC) (Figure 3-D).

Figure 3: Characteristics of GMP-grade 3D TableTrix® Microcarrier
② Industrial MSC Amplification by Automated Closed Platform
The ACISCP consists of 1-L, 5-L, 15-L bioreactors of 3D FloTrix® vivaSPIN and 3D FloTrix® vivaPREP for the purpose of cell amplification and harvesting, respectively (Figure 4-A). Bioreactors have greater economy in space. For example, 3D TableTrix® microcarriers and a 5-L bioreactor combined only use a 0.494 m2 area and are able to furnish a 9 m2 cell culture area (Figure 4-B). The cells adhere and proliferate well in bioreactors at all levels (Figure 4-C), and 2.09 × 1010 cells are finally harvested after step-by-step amplification to complete 1975-fold proliferation (Figures 4-D, 4-E).
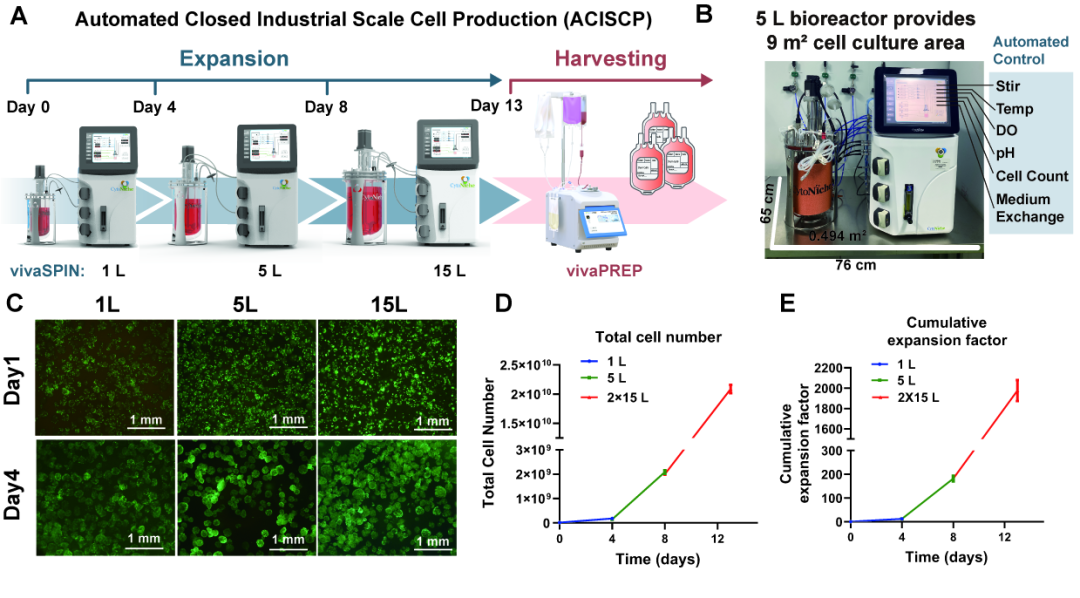
Figure 4: Industry MSC Amplification with Automated Closed Platform
③ Quality Control Indicators during ACISCP process
In each stage of ACISCP process, there are corresponding quality indicators. Cell preparations are comprehensively tested at the final preparation stage, covering sterility, safety, purity, viability, identity, stability and potency (Figure 5-A), and all the indicators are qualified. Notably, the detected quantities of both microcarrier residue and lysis residue are far below the respective allowed upper limits during three-dimensional cultivation using microcarriers. This proves that the ACISCP platform is safe and acceptable for the production of cell preparations (Figure 5-B).
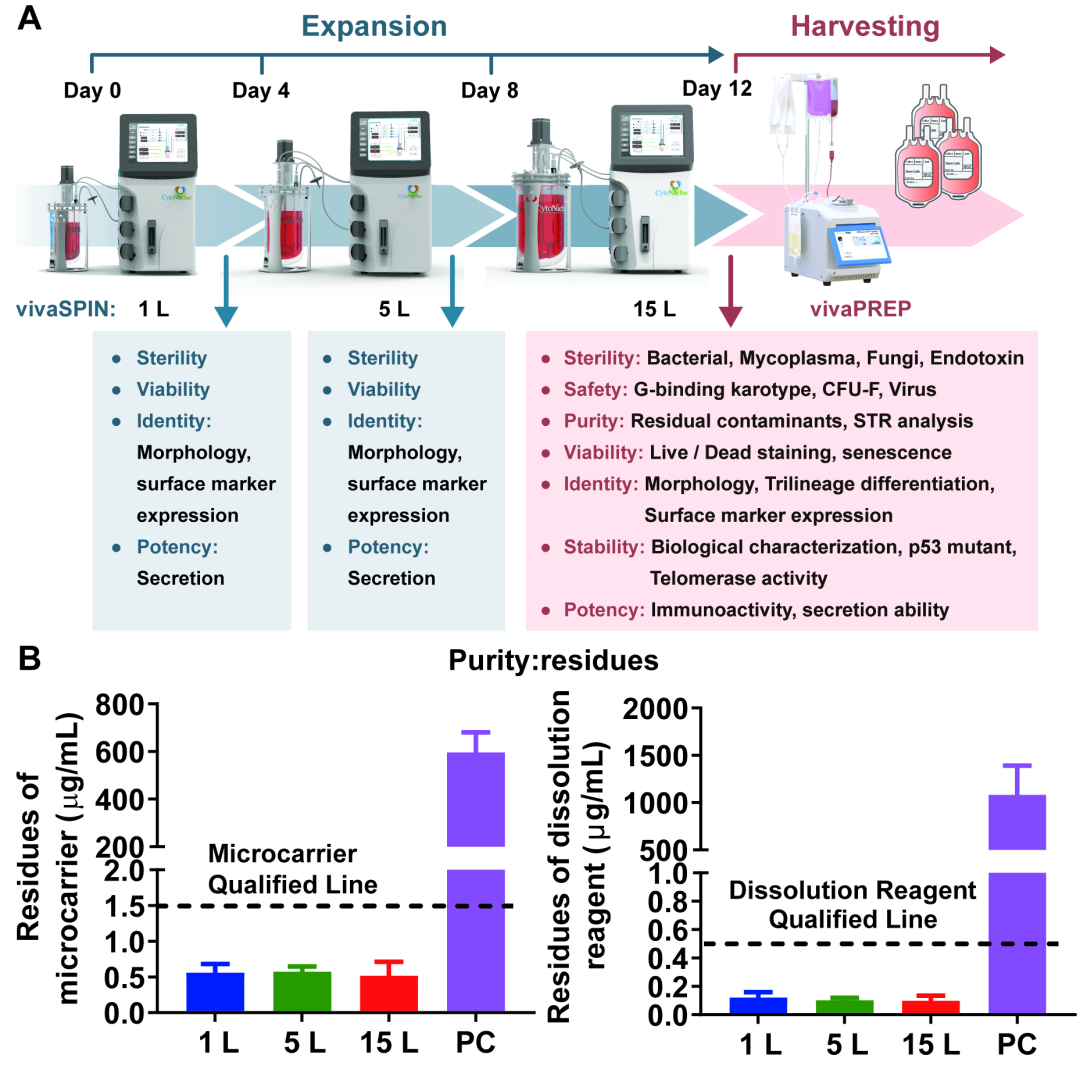
Figure 5: Quality Indicators during ACISCP-based Production
④ Potency of Harvest Stem Cells on ACISCP Platform
One key factor that determines the clinical success in the future is stem cell potency. Stem cells regulate immune responses, vascular production, and cell proliferation functions by secreting multiple factors or exosomes. In this article, the secretion of multiple factors in 2D and 3D cell supernatants are compared (Figure 6-A, 6-B). Assay of VEGF secretion by ELISA shows that 3D cultured cells secrete significantly greater amounts of VEGF than 2D cultured cells. In addition, the immunocompetence of 3D cultured cells is detected, showing that 3D amplified cells could promote the differentiation of peripheral blood mononuclear cells (PBMC) into Treg (Figure 6-C) and inhibit the differentiation into TH1 and TH17 (Figures 6-D, 6-E). This results suggest that 3D cultured cells exhibit a stronger secretory capacity and maintain MSC function in regulating immunity.
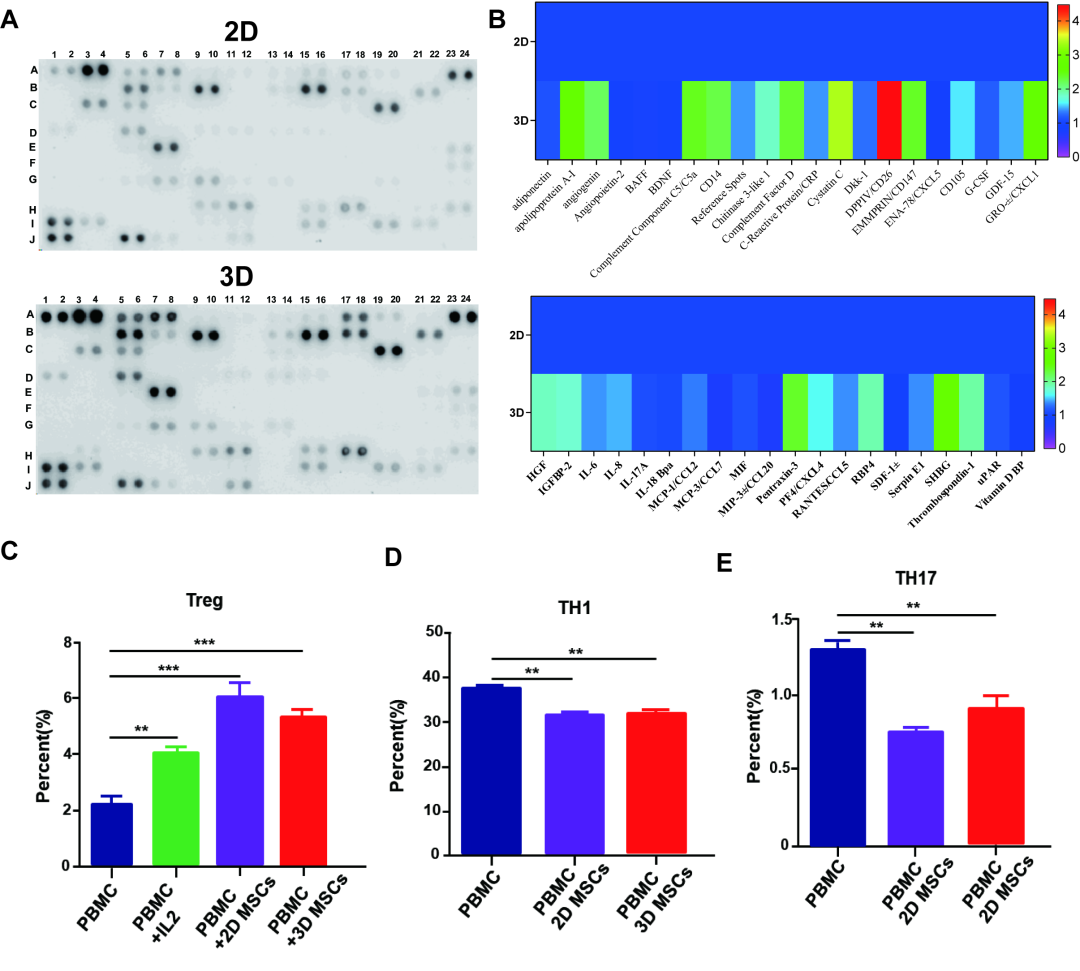
Figure 6: Potency of 3D MSCs Harvested on ACISCP Platform
【Study Content】
A brand-new automated closed industrial scale cell production (ACISCP) is created through above study to meet the needs of the cell therapy industry for cell quantity and quality, while greatly reducing labor costs, time costs and costs for instruments and consumables. The ACISCP and associated quality control standards will accelerate the industrialization development of stem cell therapy and promote cell therapy to benefit human health earlier.
【Study Team】
● This project was jointly completed by Tsinghua University, National Institutes for Food and Drug Control, Nanjing Drum Tower Hospital, Beijing CytoNiche Biotechnology Co., Ltd and Beijing Baylx Biotech Co., Ltd.
● Professor Du Ya'nan of Tsinghua University, Director Meng Shufang of National Institutes for Food and Drug Control and Director Wang Bin of Nanjing Drum Tower Hospital are the correspondence authors of this article.
● Dr. Zhang Yuanyuan from Beijing CytoNiche Biotechnology Co., Ltd, Associate Research Fellow Na Tao and Associate Research Fellow Zhang Kehua from the National Institutes for Food and Drug Control are co-first authors of this article.
【Materials and Equipment for Research and Application】
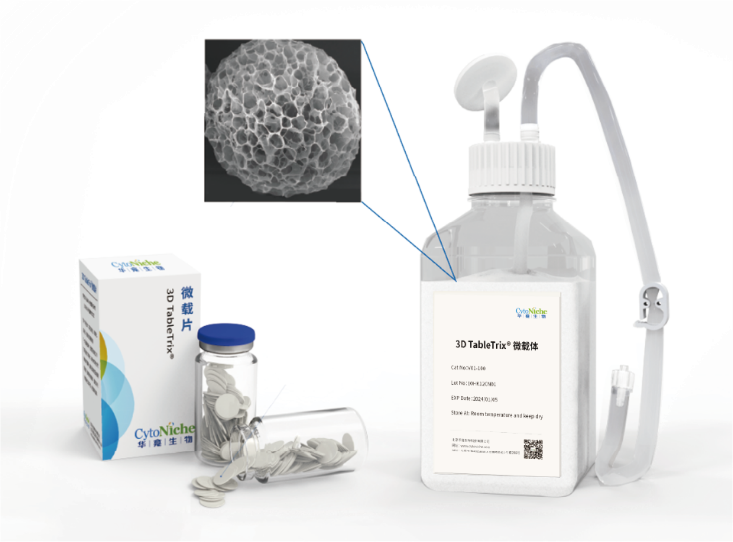
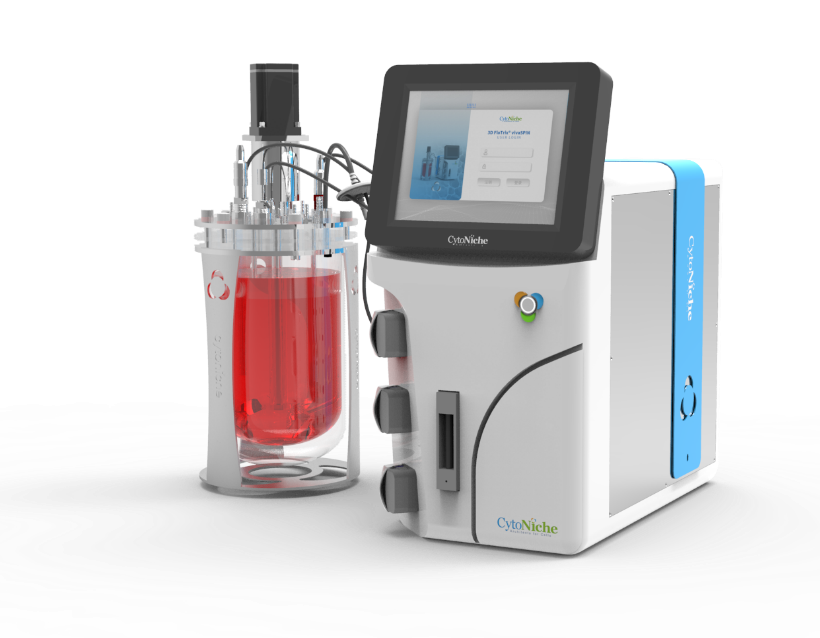
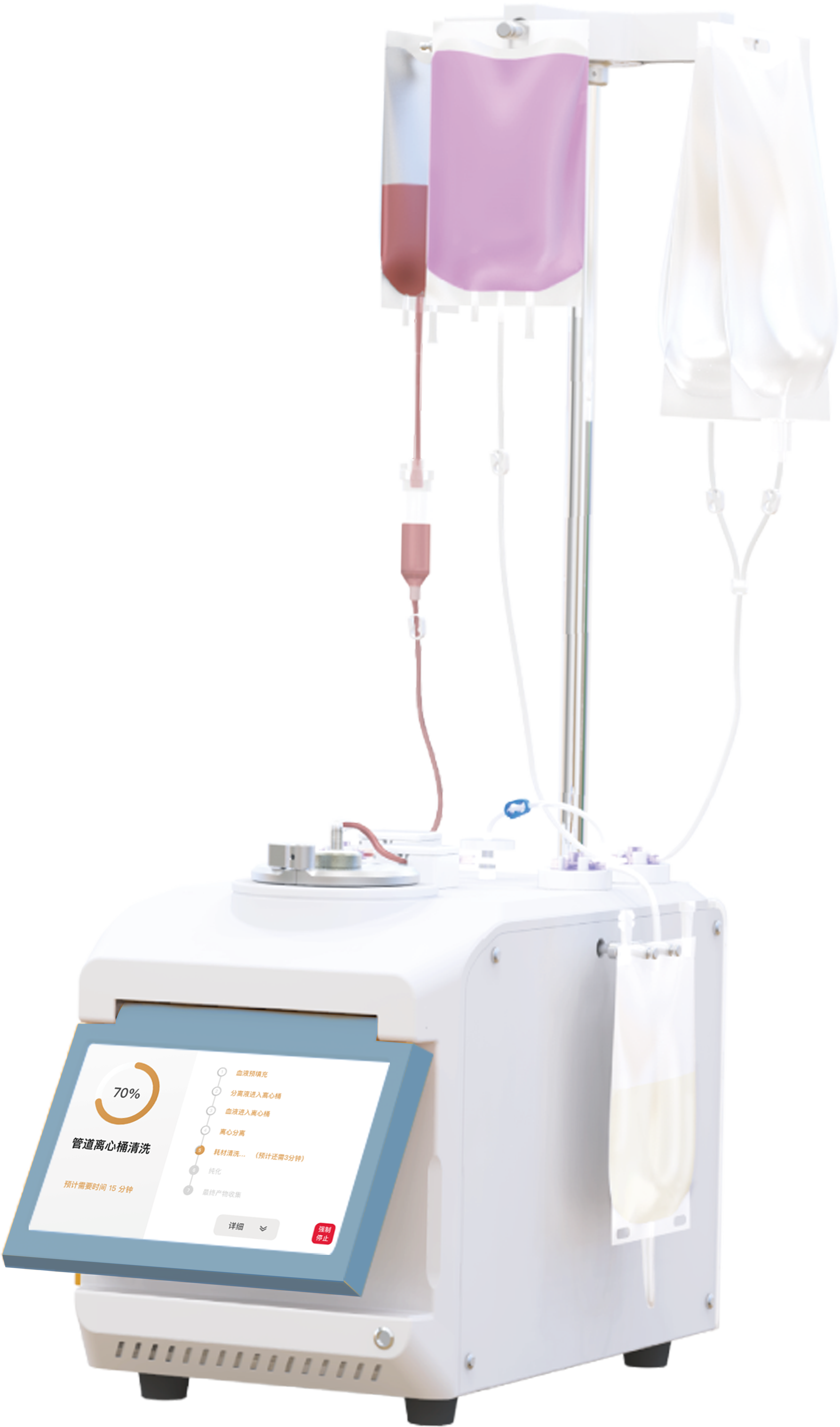
3D TableTrix® Microcarrier 3D FloTrix® vivaSPIN Bioreactor 3D FloTrix® vivaPREP Cell Harvest System
Original link
https://onlinelibrary.wiley.com/doi/10.1002/term.3341
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
