CDMO of CytoNiche Facilitates Commercialization of Cell Therapy Products | Cell Exosome Product Service
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-09-08
- Views:841
(Summary description)Exosome (EXO) is a type of microvesicles with a diameter of 30-150 nm and can maintain the biological activity of its contents in vivo, and exhibits the characteristics of low immunogenicity, high saf
CDMO of CytoNiche Facilitates Commercialization of Cell Therapy Products | Cell Exosome Product Service
(Summary description)Exosome (EXO) is a type of microvesicles with a diameter of 30-150 nm and can maintain the biological activity of its contents in vivo, and exhibits the characteristics of low immunogenicity, high saf
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-09-08
- Views:841
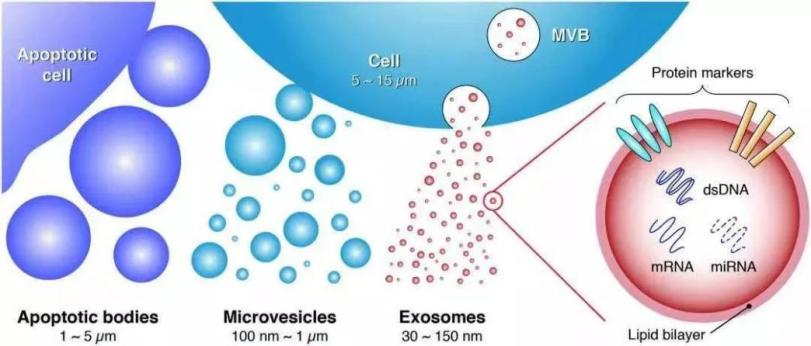
Exosome (EXO) is a type of microvesicles with a diameter of 30-150 nm and can maintain the biological activity of its contents in vivo, and exhibits the characteristics of low immunogenicity, high safety, crossing the blood-brain barrier (BBB) and other biological barriers, and plays an important role in the transmission of substances and messages among cells. Therefore, the basic research and clinical application of exosomes have seen an exponential increase in the fields of drug delivery tools, novel diagnostic markers of diseases, and therapeutic agents.
【Following problems still exist in the clinical use of exosomes】
Heterogeneity:
Almost all types of cells in the human body can produce exosomes, and even exosomes secreted by the same cell are likely to have great functional differences. The cells are amplified by in vitro culture, and the number and type of exosomes secreted each time are different, which can lead to heterogeneity of cell products between different batches.
Effect of cell preparation process:
Cell culture mode (two-dimensional culture, three-dimensional culture) and cell culture conditions (cell type, cell passage, cell density, culture time, culture medium, exosome harvest frequency) will significantly influence the quality of exosome products (yield, exosome composition and exosome biological activity, etc.). Minor changes in manufacturing conditions can have a considerable influence on the quality and activity of exosome products.
Selection and optimization of separation and purification methods for exosomes:
As of now, there is no uniform protocol for the separation and purification methods of exosomes. The separation methods used currently (such as the differential velocity ultracentrifugation method, density gradient centrifugation method, immunoaffinity method, polymer precipitation method, and ultrafiltration method) have their own advantages and disadvantages in terms of operability, treatment volume, purity and cost. The exosomes are characterized by small particle size and mix with a variety of other biomolecules, which leads to difficulty in separation and purification. Therefore, the efficient production and scalable application in the separation and purification of exosomes in large sample size is an urgent problem to be solved in the clinical application of exosomes.
Quantification protocol and quality standards for exosomes:
At present, the separated exosomes are mainly identified from three aspects:
① Exosome surface markers are identified by Western blot (WB): CD9, CD63, CD81, internal markers: TSG101, ALIX, etc.
② Analysis of morphological characteristics of exosomes by electron microscopic: Tea tray type or hemispherical shape with depression on one side;
③ The characteristics of extracellular vesicles in samples are analyzed by NTA: Particle concentration, diameter distribution, etc.
Given a lack of uniform detection methods and standardized technical specifications for exosomes, the validity, reproducibility, and universality of exosome preclinical findings are still unanswered questions based on the characteristics of exosome heterogeneity. Therefore, the quality evaluation on exosomes should not only be limited to the detection process, but also consider the selection of cells or tissue samples before detection. Also, it is necessary to develop a rapid and high-throughput detection method at the single-particle level for quality monitoring.
Conditions and methods for storing exosomes:
The integrity and biological activity of extracted exosomes can be affected by factors such as storage medium, storage temperature and duration. Exosomes are generally suspended in phosphate buffer after extraction. The conventional exosome storage temperatures in the laboratory are generally 4 °C, -20 °C and -80 °C; -20 °C or lower is a relatively desirable long-term storage condition for exosomes. Exosomes should be systematically studied for quality stability of storage conditions and methods according to the direction of the clinical translational application, source, dosage form, and mode of administration.
Tissue selection and in-vivo biological distribution:
The outcome, biodistribution, pharmacokinetics, and specificity of target delivery organ distribution of exosomes after entry into recipient cells as well as their therapeutic effects on diseases have not been fully elucidated.
The exosomes from different sources and different administration methods have homing ability of different tissue tendencies in vivo, which might be related to their membrane surface receptors and extracellular matrix-binding proteins; Since exosomes are naturally released by cells in vivo, cells in vivo can also be engineered to release engineered exosomes in vivo, and exosome surface proteins or chemical structures can be engineered to enrich them in specific tissues and organs.

【In response to the above challenges, CytoNiche Biotechnology can provide the following services】
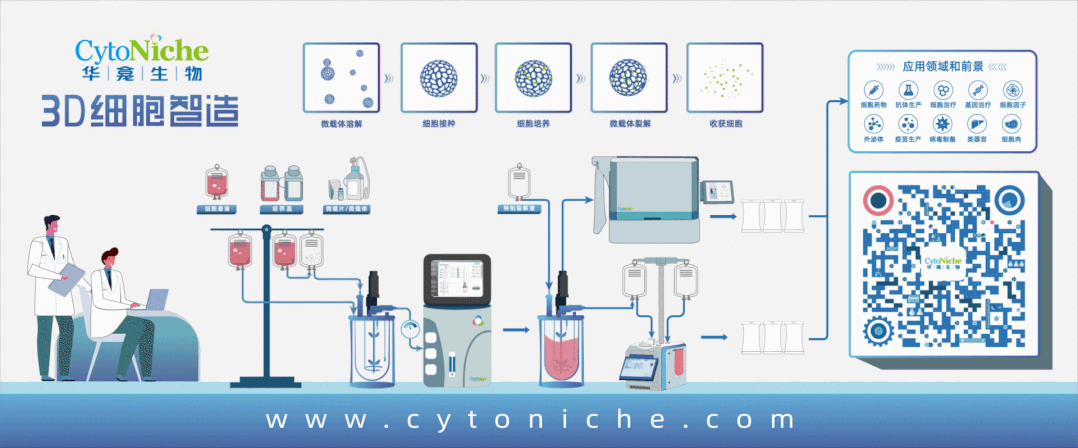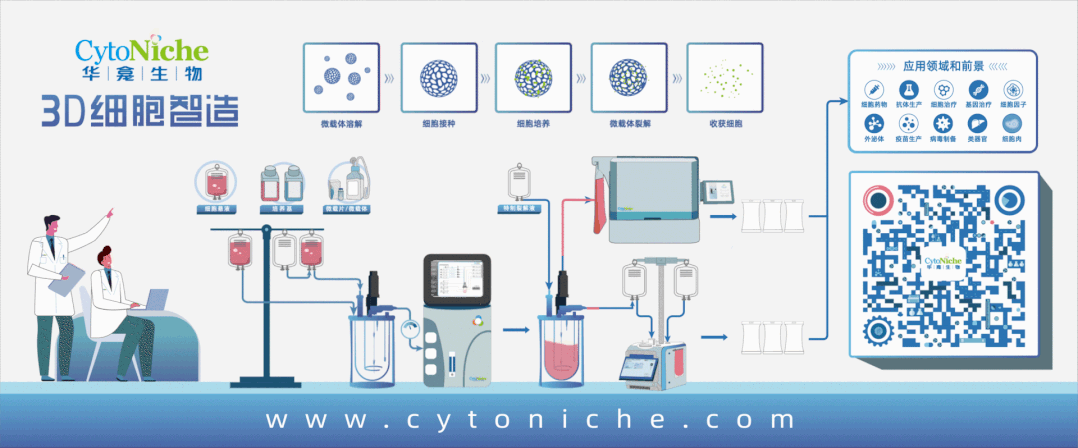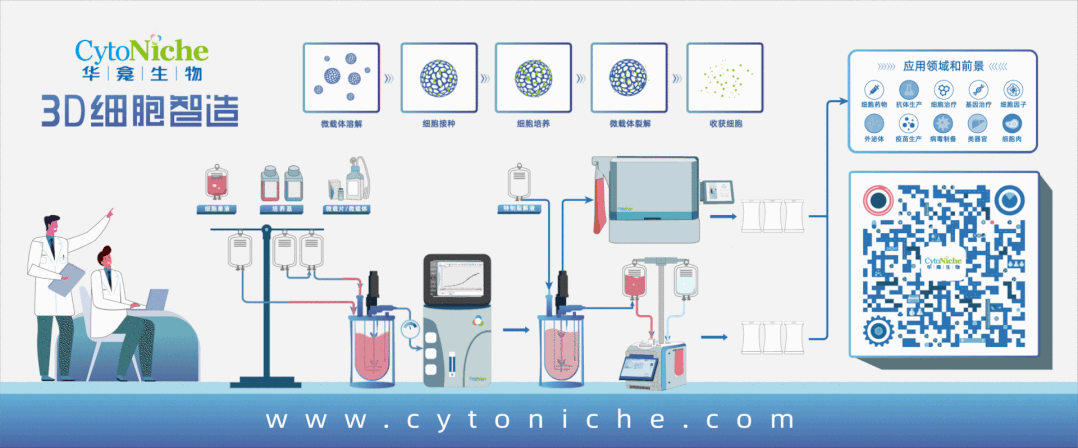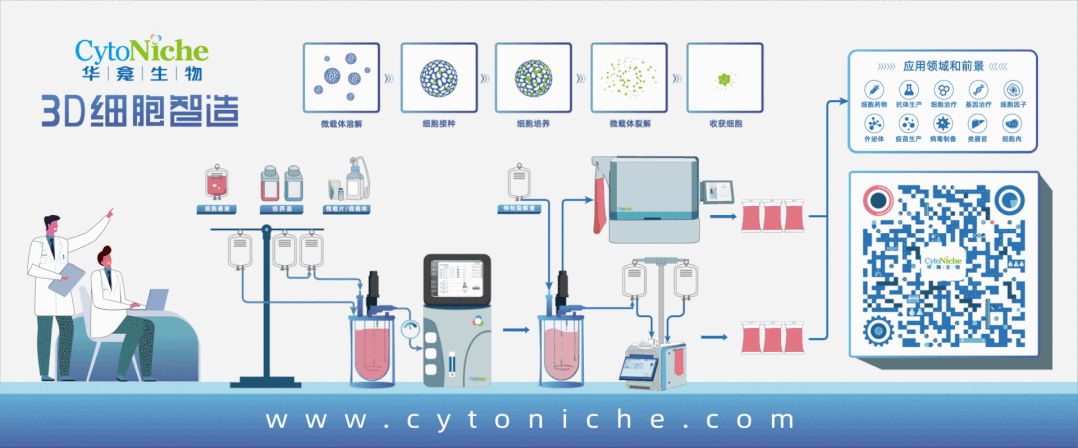
1. Large-scale and standardized preparation of exosomes
Mesenchymal stem cell exosomes (MSC-Exos) have hit the subject for clinical translational applications based on their effects in promoting tissue repair and regeneration such as inhibiting inflammatory responses, activating anti-apoptotic and pro-survival pathways. However, the production for pharmaceutical GMP-grade MSC-Exos remains a major challenge in the industry.
Two key points for the large-scale application and development of MSC-Exos are:
① The scale-up of the MSCs-based culture mode.
② The improvement of exosome separation-based purification methods.
Compared with the MSC cultured with the conventional 2D culture method, CytoNiche Biotechnology 3D TableTrix® microcarriers and 3D FloTrix® series bioreactors exhibit higher exosome secretion.
The 3D process has the following advantages in the production of MSC-Exos:
① The continuous culture process can be achieved to avoid the accumulation of metabolic by-products in the bioreactor through continuous medium perfusion so that exosomes can be continuously harvested at different time points.
② Mesenchymal stem cells that meet the specifications for stem cell therapy can be harvested in real time to ensure the quality of exosomes.
③ Single batch can be realized, and large-scale exosome products can be harvested at the same time to ensure the homogeneity of exosome quality.
④ Combined with the 3D FloTrix® vivaEXO exosome harvest system, 10 L cell culture supernatant can be concentrated to 500 mL within a few hours to efficiently harvest exosome products.
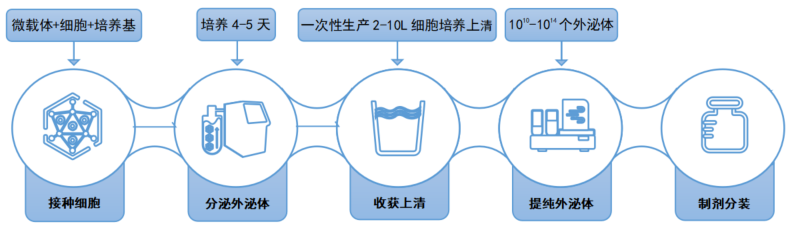
Flow Chart of Exosome Production and Purification
1. Separation and identification of exosomes
According to the Minimal Information for Studies of Extracellular Vesicles (MISEV2018) proposed by International Society of Extracellular Vesicles (ISEV) in 2018, and the group standard of Mesenchymal Stem Small Cell-derived Extracellular Vesicles proposed by Chinese Research Hospital Association, the identification for human mesenchymal stem cell extracellular vesicles (EVs, including exosomes) includes the following contents:
① Morphology: Tea tray or cup structure with clear membrane structure and without agglomeration should appear under transmission electron microscope, with clear edges.
② Particle size: Particle diameter should be distributed within the range of less than 200 nm, and there shall be a peak particle size within the range.
③ Marker proteins: Any two of the small extracellular vesicle surface markers CD9, CD81 and CD63 are positively expressed; any one of the small extracellular vesicle's internal markers TSG-101 and Alix is positively expressed.
④ Purity: GM130 or Calnexin, a negative marker of small extracellular vesicles, should be negative; the ratio of the number of small extracellular vesicles to the amount of protein should be not less than 1 × 108 particles/microgram protein.
⑤ Immunomodulation: Co-incubation of small extracellular vesicles derived from human mesenchymal stem cells with peripheral blood mononuclear cells derived from healthy individuals can inhibit their proliferation and inhibit Th1 cell function.
⑥ Microorganism: HIV, HBV, HCV, HTLV, HEBV, HCMV, TP, fungi, bacteria and mycoplasma should be negative.
【The following services are provided by CytoNiche Biotechnology】
① Quality identification of large-scale cultured cells.
② The exosome particle concentration and particle size range are detected by Nanoparticle Tracking Analyzer (NTA).
③ 3D exosome surface marker expression is detected by Western Blotting (WB).
④ 3D exosome structures are observed by Transmission Electron Microscopy (TEM).
⑤ Detection of microorganisms, endotoxin, mycoplasma, protein concentration, and cell secretory factors in cell culture supernatants.
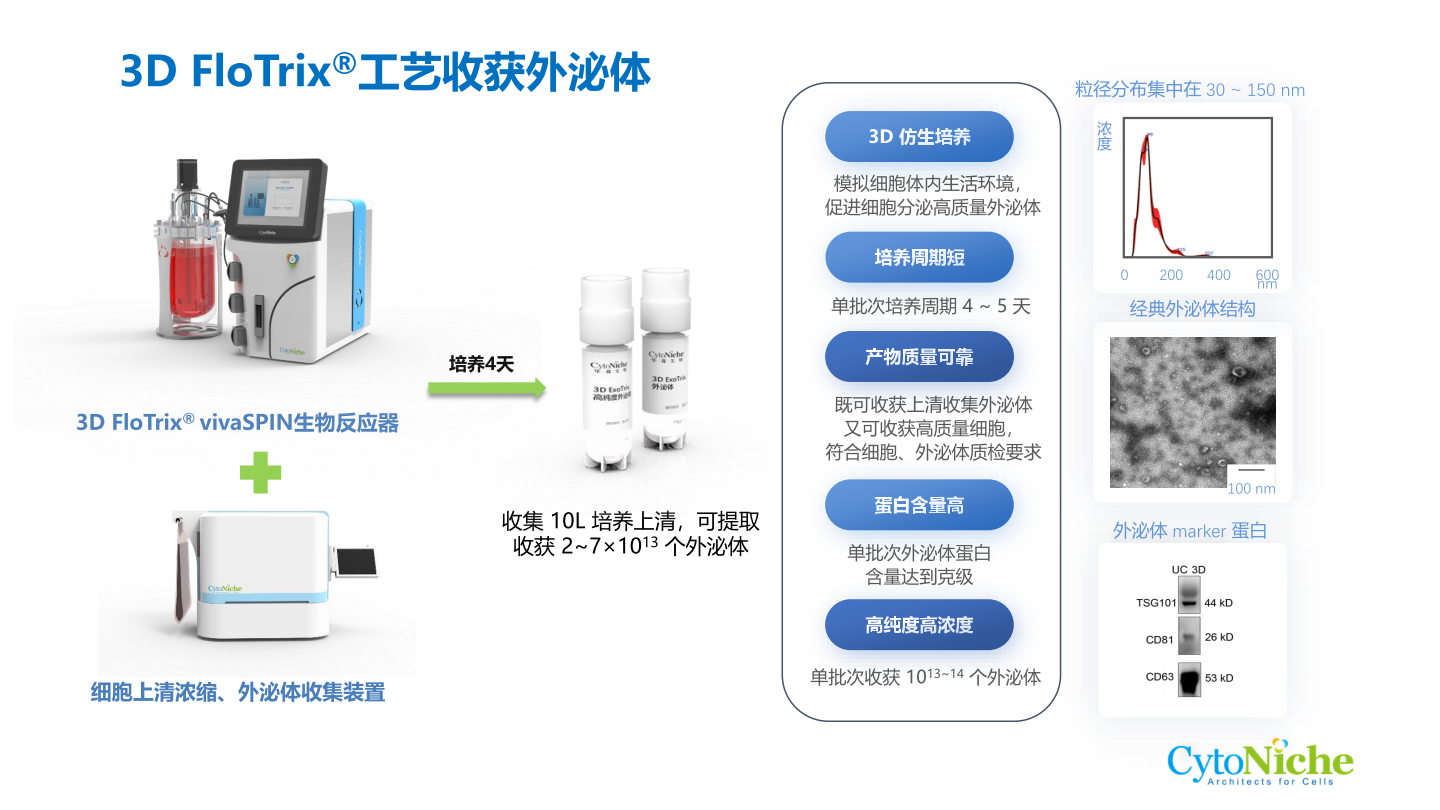
To date, there are inconsistencies in the clinical use of therapeutic exosomes, including all areas of exosome production, such as methods for exosome separation, quantification, and characterization. The standardized and simplified preparation processes in large-scale exosome production are key to the reproducibility of exosome therapy product results in preclinical and clinical settings.
Reference:
1. Group Standard for Small Extracellular Vesicles Derived from Human Mesenchymal Stem Cells (Chinese Research Hospital Association)
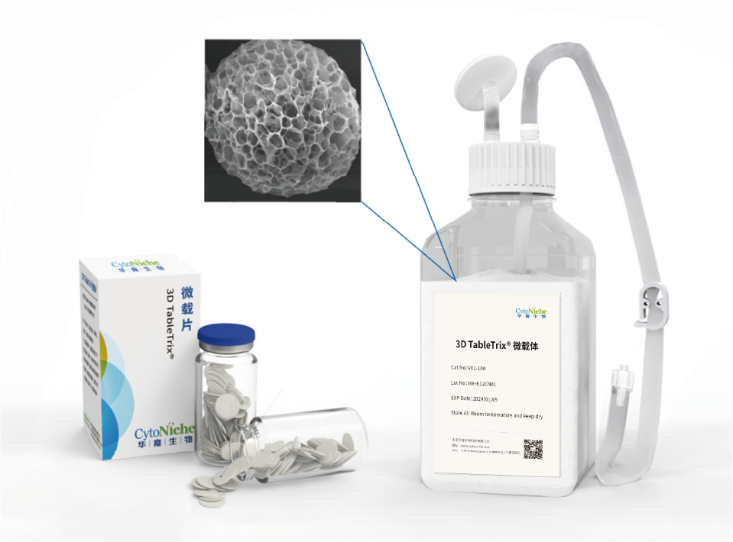
✦ 3D TableTrix®
Cell microcarrier
• Has obtained the qualification for pharmaceutical excipients from FDA (DMF) and CDE.
• Elastic porous structure with large specific surface area
• 3D biomimetic, customizable
• Specific degradation technology
• Aseptic tablet packaging, no need for pretreatment

✦ 3D FloTrix®
Three-dimensional stem cell culture medium
• Customized for 3D mesenchymal stem cell culture process
• GMP-grade production
• Serum-free, animal component-free
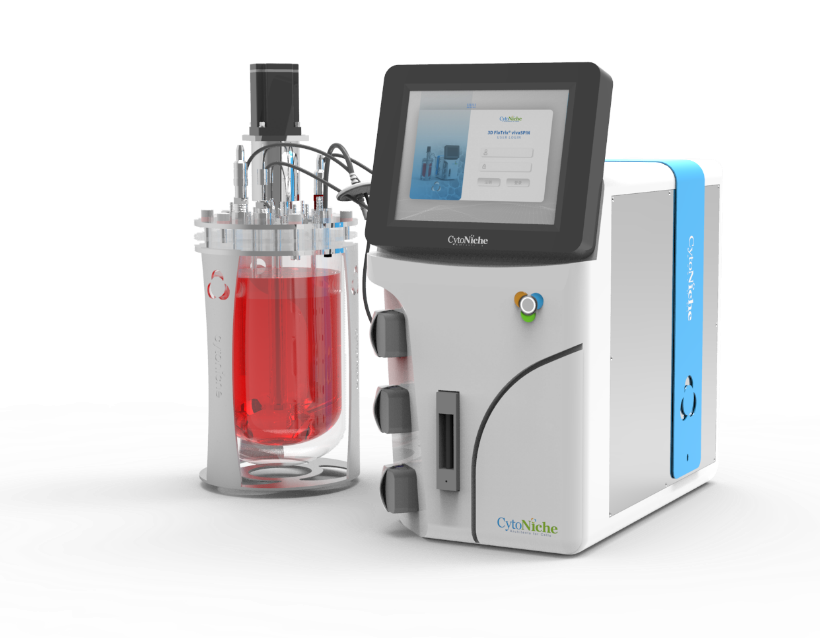
✦ 3D FloTrix®vivaSPIN
Automated bioreactor
• Implementation of 3D cell real-time online counting
• High precision mass flowmeter, enabling reduction in bubble generation, suitable for 3D cell dynamic culture process
• 3D TableTrix® cell microcarrier dedicated supernatant filtration system
• Audit trail function available, providing 3Q certification and complying with GMP
✦ 3D FloTrix®vivaSPIN
Automated bioreactor
• Implementation of 3D cell real-time online counting
• High precision mass flowmeter, enabling reduction in bubble generation, suitable for 3D cell dynamic culture process
• 3D TableTrix® cell microcarrier dedicated supernatant filtration system
• Audit trail function available, providing 3Q certification and complying with GMP

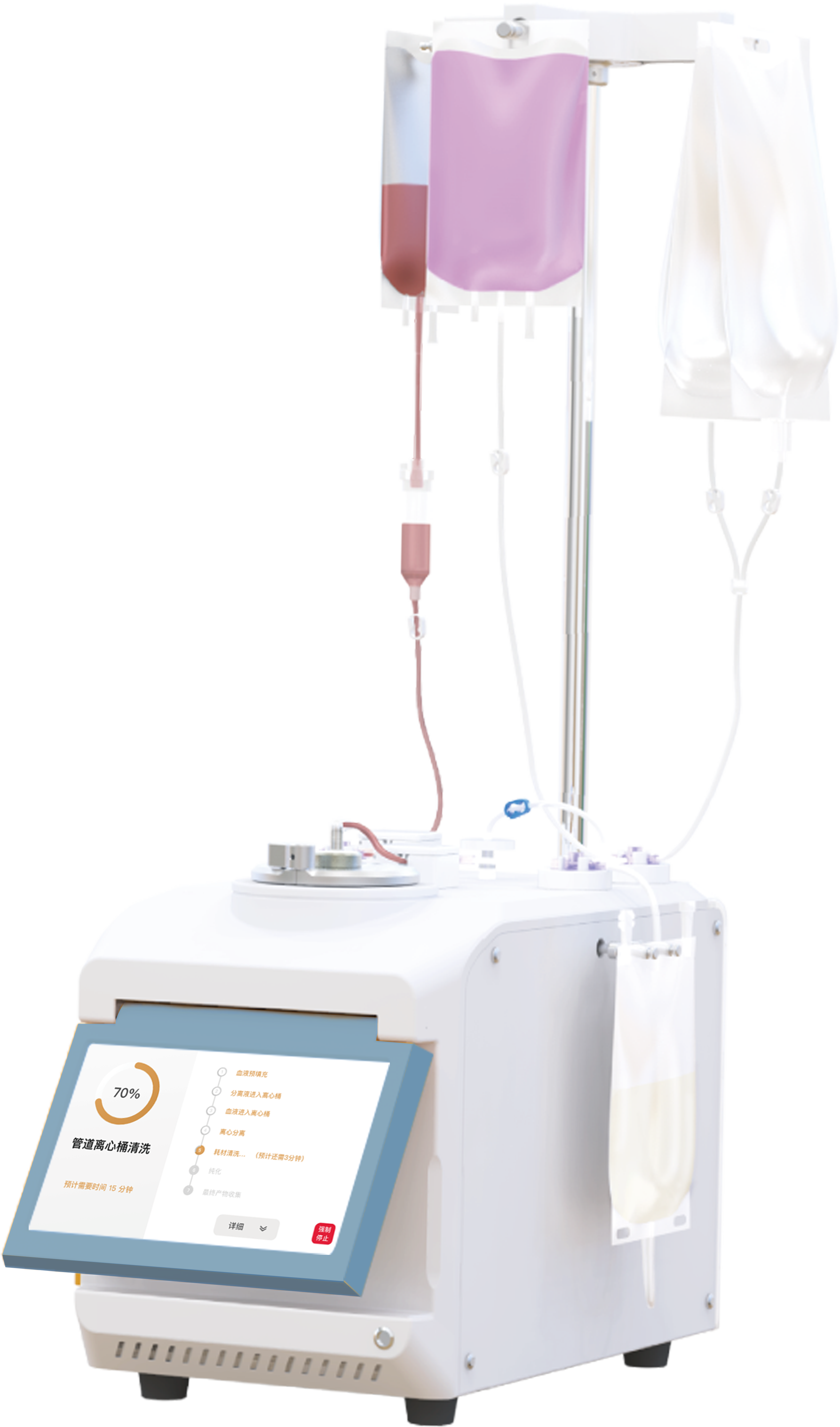
✦ 3D FloTrix®vivaEXO
Exosome harvesting device
• Capable of concentrating 10L of stock solution into less than 500mL in one single batch in 1-3 hours
• Adopting disposable fully enclosed consumable packs that are ready out of the box
• Supports setup and display of various parameters in the running process in real time.
[CytoNiche]
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
