Strategic cooperation | CytoNiche Starts In-depth Cooperation on CDMO Project of Cell Innovation Drugs with Beizheng Stem Cell.
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-30
- Views:590
(Summary description)Recently, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as CytoNiche) and Jiangxi Beizheng Stem Cell Biotechnology Co., Ltd. (hereinafter referred to as Beizheng Stem Cell) held a strategic cooperation signing ceremony. Through this strategic cooperation, the two parties will make full use of their respective advantages and promote the research and development in the field of large-scale and homogeneous culture of stem cells and clinical application therapy, which is another case for both parties to practice powerful cooperation and innovative development.
Strategic cooperation | CytoNiche Starts In-depth Cooperation on CDMO Project of Cell Innovation Drugs with Beizheng Stem Cell.
(Summary description)Recently, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as CytoNiche) and Jiangxi Beizheng Stem Cell Biotechnology Co., Ltd. (hereinafter referred to as Beizheng Stem Cell) held a strategic cooperation signing ceremony. Through this strategic cooperation, the two parties will make full use of their respective advantages and promote the research and development in the field of large-scale and homogeneous culture of stem cells and clinical application therapy, which is another case for both parties to practice powerful cooperation and innovative development.
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-08-30
- Views:590
Recently, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as CytoNiche) and Jiangxi Beizheng Stem Cell Biotechnology Co., Ltd. (hereinafter referred to as Beizheng Stem Cell) held a strategic cooperation signing ceremony. Through this strategic cooperation, the two parties will make full use of their respective advantages and promote the research and development in the field of large-scale and homogeneous culture of stem cells and clinical application therapy, which is another case for both parties to practice powerful cooperation and innovative development.

Beizheng Stem Cell has gathered an expert research team composed of influential domestic academicians, professors listed in the national "Thousand-talent Plan" and foreign experts, which provides a solid foundation for the research of life science, the research, development and industrialization of regeneration medicine application technology. Based on its profound technical accumulations and complete service system in relevant fields, Beizheng provides safe and effective stem cell technologies and biological components for various clinical products and derivatives for clinical therapy, as well as biological materials compatible with human body and tissue engineering products for repair and regeneration.
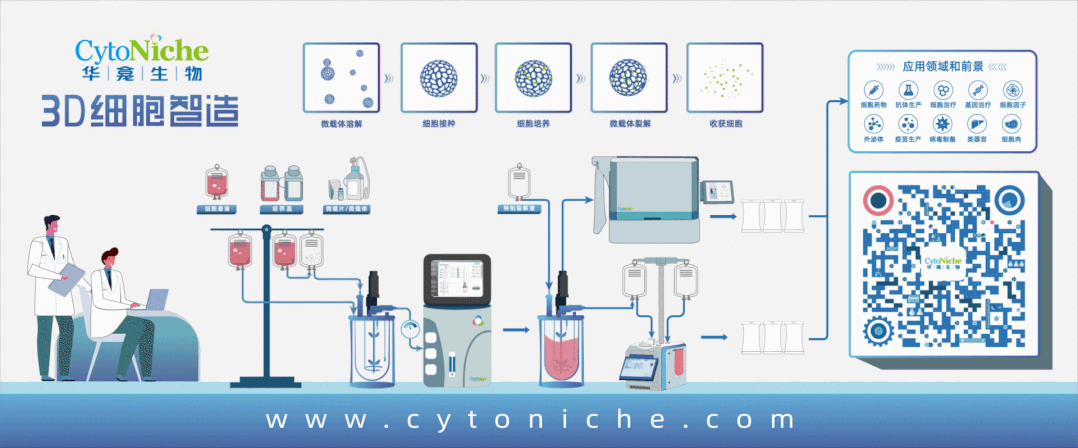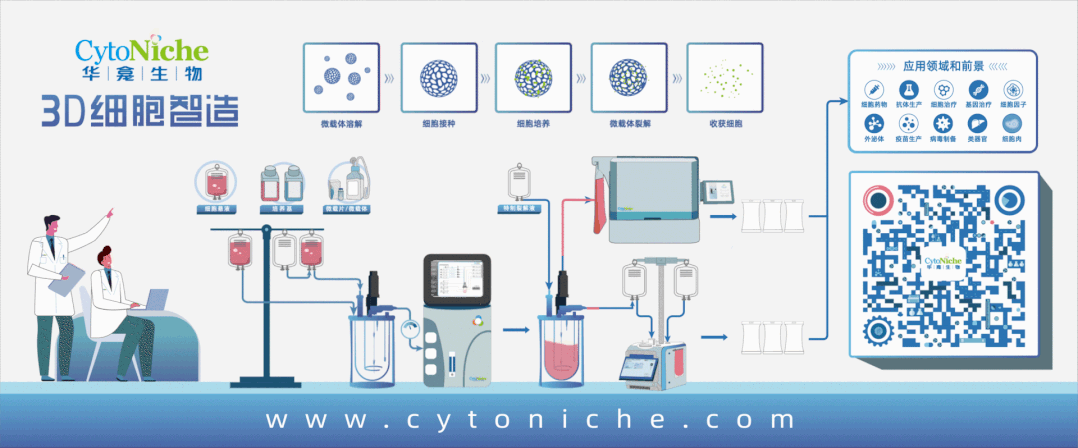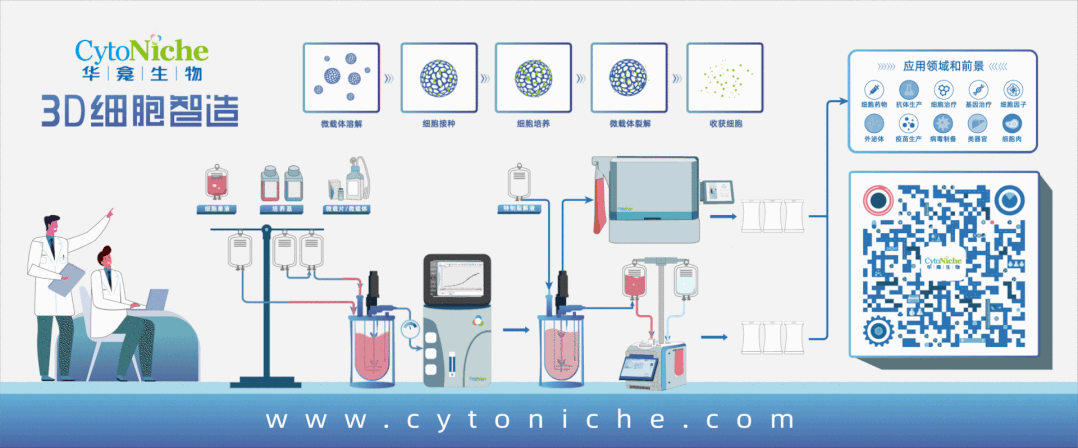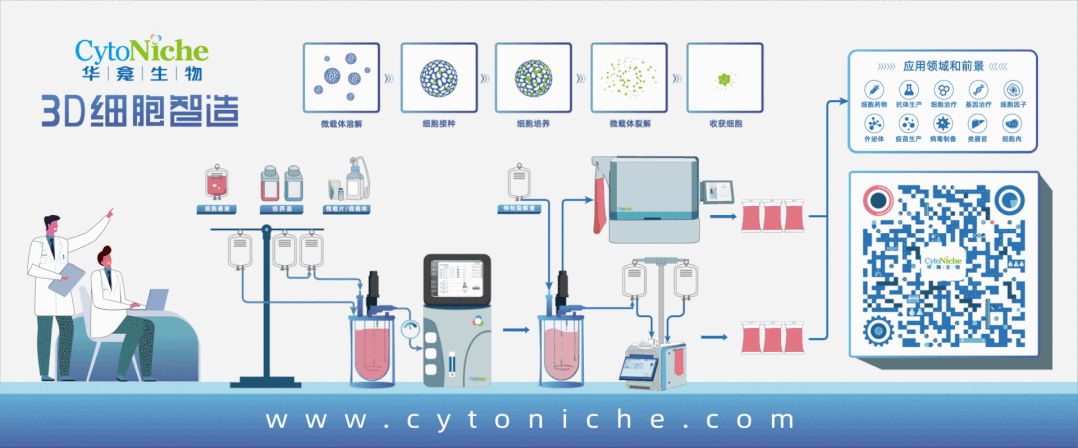
CytoNiche has built the 3D FloTrix® Cell Technology Platform. Based on its unique degradable microcarrier technology and 3D cell preparation process, it has solved a series of current bottleneck problems in the industrial development of cell therapy products, helped many enterprises to set up totally-enclosed automatic cell drug production lines, thus realizing large-scale, automatic, standardized and intelligent production and preparation of cell drugs and their derivatives. CytoNiche provides CDMO services different from the existing CGT cell preparation processes, including technical research, process development and GMP production services that cover large-scale production and preparation of mesenchymal stem cells (MSC), exosomes and viruses from different tissue sources, to facilitate product transformation and clinical application of innovative achievements.
CytoNiche and Beizheng Stem Cell work together to integrate superior resources, break through technical bottlenecks through microcarrier 3D cell application technology, create innovative products around stem cell therapy R&D projects, and provide stem cell therapy solutions with standardized quality to solve the previously unsatisfied clinical needs faced by mankind.

Cooperation for mutual benefits
Mr. Zhao Yong, General Manager of Beizheng Stem Cell, Dr. Liu Wei, General Manager of CytoNiche, Dr. Sun Yanxun, Director of Cell Transformation Center, Mr. Wang Zhiqian, Director of Marketing Center and other representatives of both parties jointly attended the signing ceremony.
【About Beizheng Stem Cell】
Beizheng Cell (Beijing) Biotechnology Co., Ltd originated in 2008, focusing on the industrial chain of stem cell and immune cell technology. Its headquarters is located in the core area of Beijing Zhongguancun Life Science Park - Peking University Medical Industry Park. It is where the largest cell repository in northern China is established.


Left: Headquarters: Zhongguancun Life Science Park-Peking University Medical Industry Park
Right: Jiangxi Branch in Ganzhou International Enterprise Center
Beijing and Jiangxi join forces to develop together with the revolutionary area. Beizheng Stem Cell established a tissue sample bank in Jiangxi in 2017, and passed the joint review of four national departments in 2021, officially starting to operate the cell preparation center in southern Jiangxi. Meanwhile, the construction of the cell preparation center in central Jiangxi was started. Beizheng Stem Cell is committed to becoming the basic resource platform that can give a powerful boost for the development of the entire industry. In strict accordance with relevant national regulations and technical specifications, Beizheng has built an industrial production base for cell storage and preparation, R&D and application, and medical instrument registration.


Southern Jiangxi Cell Preparation Center and Self-Built Laboratory
With the technical support of the Cell Biology discipline in the School of Basic Medical Sciences, Peking University, Peking University Stem Cell Research Center, Peking University Systems Biology Research Institute, and experts in the research of hematopoietic reconstitution and immune reconstitution of radiation sickness from the Academy of Military Medical Sciences, Beizheng has been providing stem cell technology, immune cell technology and products, cell-related biological products, biomaterials compatible with human body, and tissue engineering products for repair and regeneration for clinical therapy for a long time. Beizheng has obtained a number of domestic and international patents related to stem cell and immune cell technologies.

Part of the patent certificates obtained by Beizheng
In strict accordance with the guiding principles of National Health Commission of the People's Republic of China and National Medical Products Administration on the quality control and preclinical research of stem cell preparation, the company has built cell engineering and R&D bases in terms of biological tissue sample resource storage, large-scale cell preparation, cell drug R&D, and clinical application research. It is a member of China Clinical Translational Medicine Industry Society of National Association of Health Industry and Enterprise Management, a Chinese scientific and technological innovation unit recognized in the 70th anniversary of the founding of the People's Republic of China, and one of the top ten leading enterprises in China's health industry in 2019.
【About CytoNiche】

Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. Their core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D "intelligent manufacturing" platform for cells, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
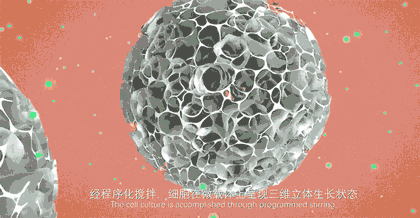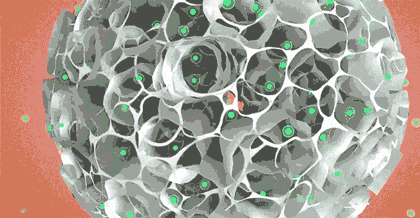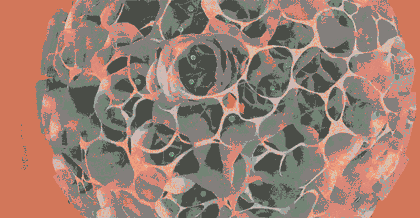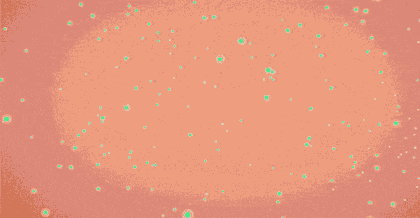
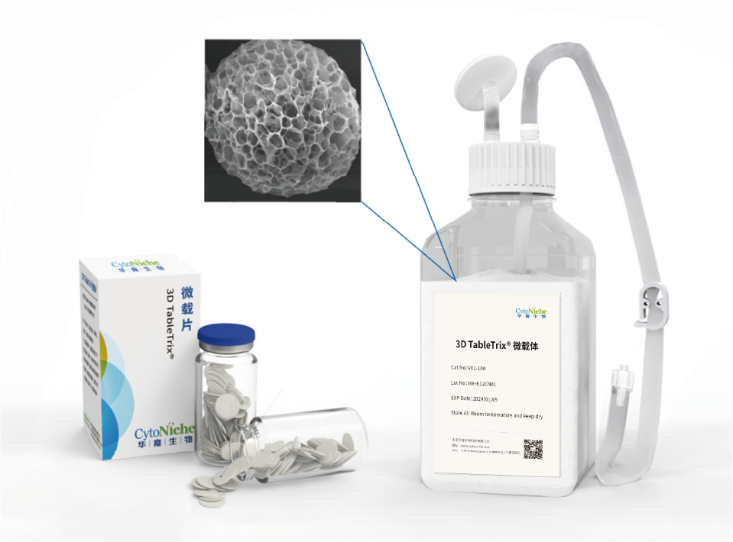
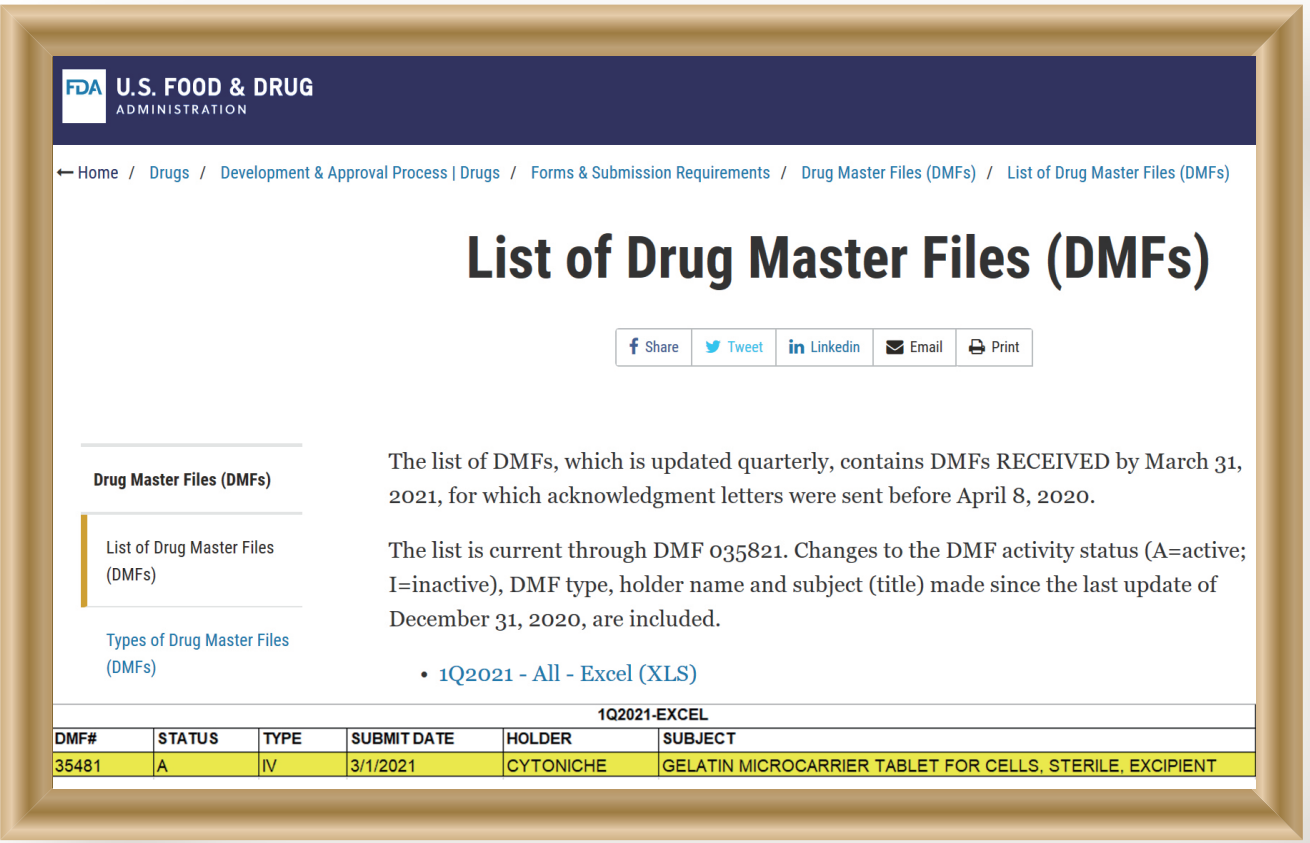
The core product of CytoNiche, 3D TableTrix® Microcarrier (microcarrier), is an independent innovation and the first pharmaceutical excipient microcarrier that can be used for cell drug development. CytoNiche has passed the inspection of the National Institutes for Food and Drug Control and other relevant authorities, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). In addition, the product has obtained the DMF qualification for pharmaceutical excipient from FDA (DMF: 35481).

The products and services of CytoNiche can be widely applied in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines and protein products. They also have broad application prospect in regenerative medicine, organoid and food technology (cell-cultured meat, etc.).




Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
