[3D Microcarriers] Application in the Field of Vaccines — MDCK Cells
- Categories:Company News
- Author:
- Origin:
- Time of issue:2022-07-14
- Views:677
(Summary description)Influenza is a highly contagious disease, and inactivated influenza vaccine and subunit vaccine are currently the most widely used types of vaccines on a commercial scale. Influenza vaccines consist of one or more virus strains which have been conventionally produced in the allantoic cavity of embryonated hens’ eggs.
[3D Microcarriers] Application in the Field of Vaccines — MDCK Cells
(Summary description)Influenza is a highly contagious disease, and inactivated influenza vaccine and subunit vaccine are currently the most widely used types of vaccines on a commercial scale. Influenza vaccines consist of one or more virus strains which have been conventionally produced in the allantoic cavity of embryonated hens’ eggs.
- Categories:Company News
- Author:
- Origin:
- Time of issue:2022-07-14
- Views:677
[Progression of Influenza Vaccine]
Influenza is a highly contagious disease, and inactivated influenza vaccine and subunit vaccine are currently the most widely used types of vaccines on a commercial scale. Influenza vaccines consist of one or more virus strains which have been conventionally produced in the allantoic cavity of embryonated hens’ eggs.
However, producing vaccines from chicken embryos has many disadvantages:
a. Selective reproduction of virus leads to low immunogenicity;
b. High labor intensity and low yield, and it is difficult to meet the demand during an influenza pandemic;
c. Antigenic differences;
d. If the influenza virus is of avian origin, egg-derived vaccines may be lethal for human;
e. Egg-derived components may cause fever and pathogenicity in recipients [1].
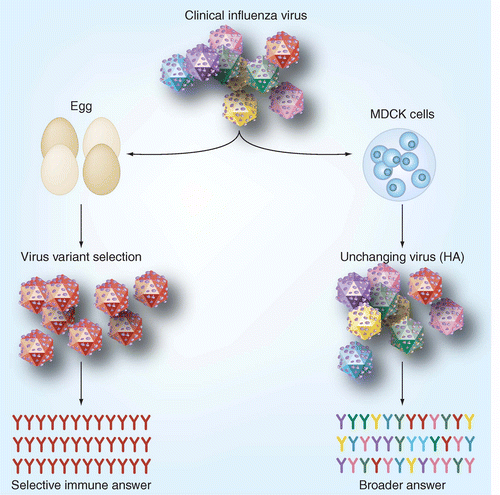
Figure 1. Virus selection in eggs versus authentic copy in MDCK cells [2]
Cell-cultured influenza viruses have many advantages:
a. High immunogenicity;
b. Easier scale-up for production, lower labor intensity, faster preparation, and high yield;
c. Closed production without bacterial contamination;
d. Standardized preparation with higher initial purity;
e. Standardized process to reduce inter-batch variation;
f. Provides an option for vaccinating people who are allergic to eggs.
[Application of MDCK Cells for Vaccines]
MDCK cells are considered to be one of the most suitable cell lines for the production of influenza A and B vaccines because of their high affinity to viruses, rapid proliferation rate, and resistance to mutations. MDCK originally requires adherent culture, but traditional two-dimensional planar culture is not conducive to gradual scale-up. With the discovery and use of microcarriers, this problem is gradually solved. Microcarriers can realize suspension culture of adherent cells, which can effectively increase the amount of cell culture and virus harvesting in one batch.
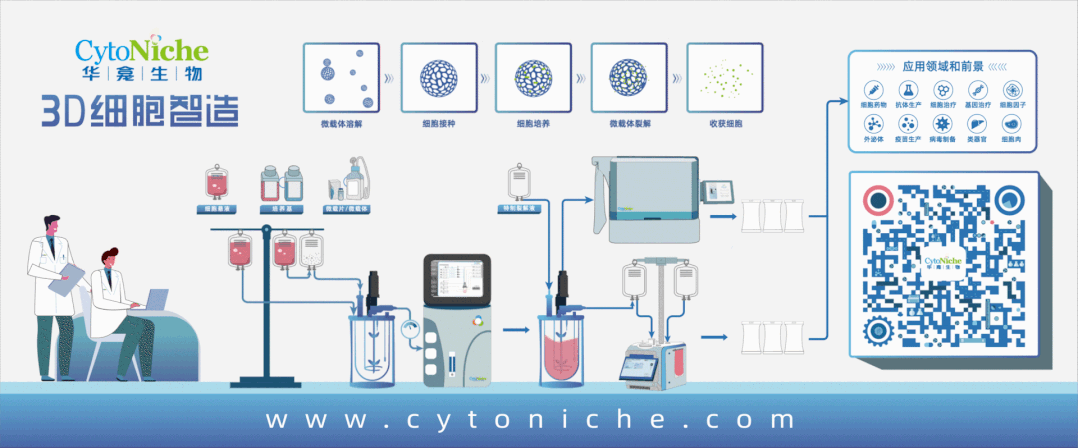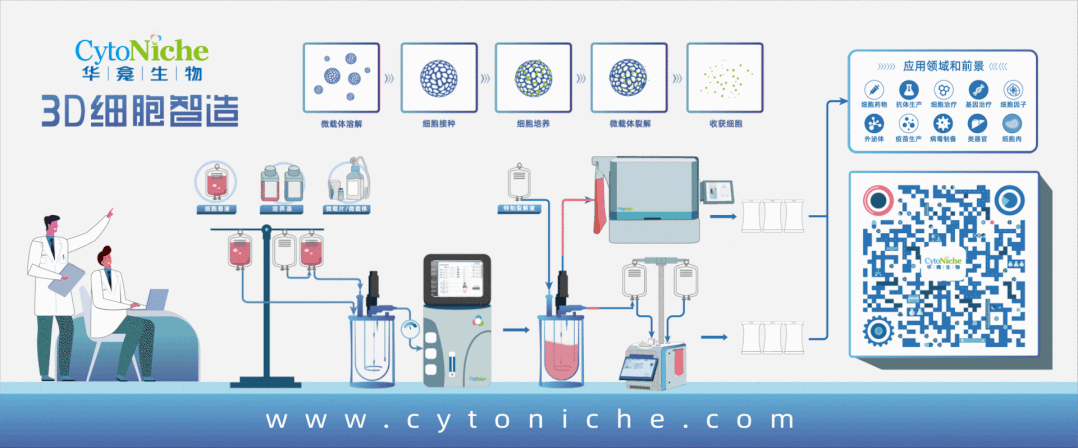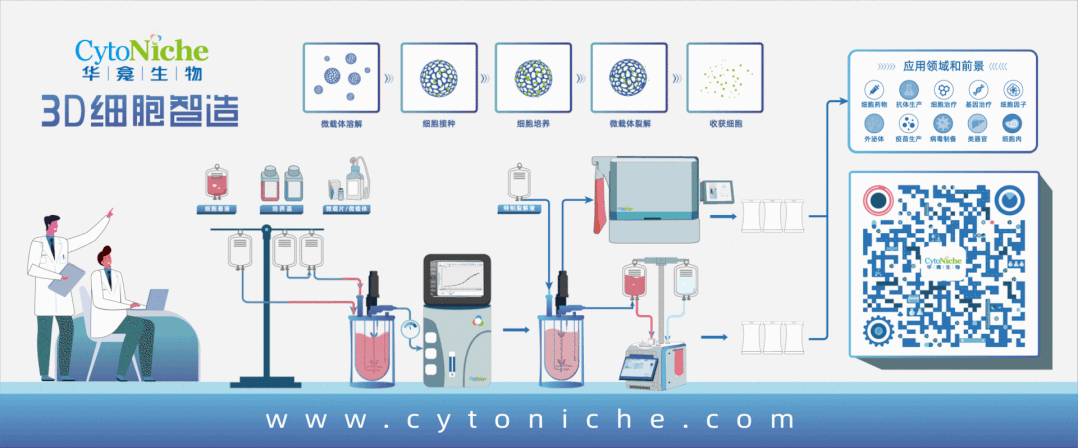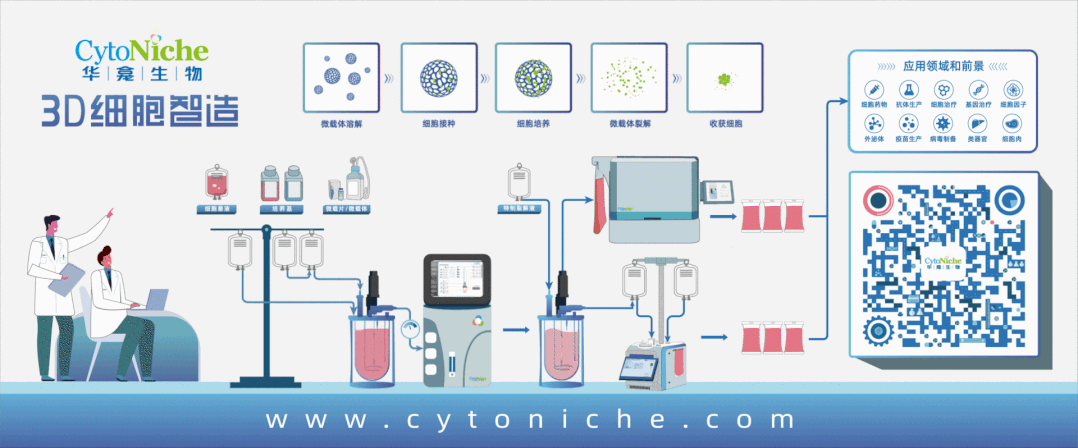
[Introduction of MDCK Cell Culture Process with 3D TableTrix® Microcarriers]
For adherent MDCK, the use of a microcarrier/reactor process is currently the best option for large-scale culturing of cells needed for influenza virus transmission because of their high specific surface area. Bioreactors can be used for large-scale culture and can be better monitored and controlled.
The microcarrier suspension culture technique can be carried out in a stirred bioreactor. The application of 3D TableTrix® microcarriers (Figure 2) enables cell breeding in the 3D FloTrix® miniSPIN bioreactor (Figure 3). Combined with the 3D FloTrix® vivaSPIN automated bioreactor (Figure 4), the cell volume can be scaled up gradually to complete the harvesting of cells, viruses, and cell products. Cell culture process parameters such as temperature, dissolved oxygen, pH, rotational speed, Air, O2, CO2, N2 can be controlled with high precision and automation, providing a good environment for cells to facilitate cell proliferation. By setting up an automatic medium exchange program (Table 1), sufficient nutrients were provided for cell growth and a favorable growth environment was maintained, eventually reaching a cell density of nearly 10 million/mL (Figure 5).
Compared with two-dimensional culture, the cost of space, labor, reagent consumables, and time is reduced when culturing cells in large quantities, while the cell density is greatly increased. Adherent cultures based on 3D TableTrix® microcarriers have now achieved cell densities similar to those of full suspension cultures [3]. And compared with suspension cell culture, adherent culture has the distinct advantage of preventing potential tumorigenicity of suspension cells and reducing cell loss during virus infection.
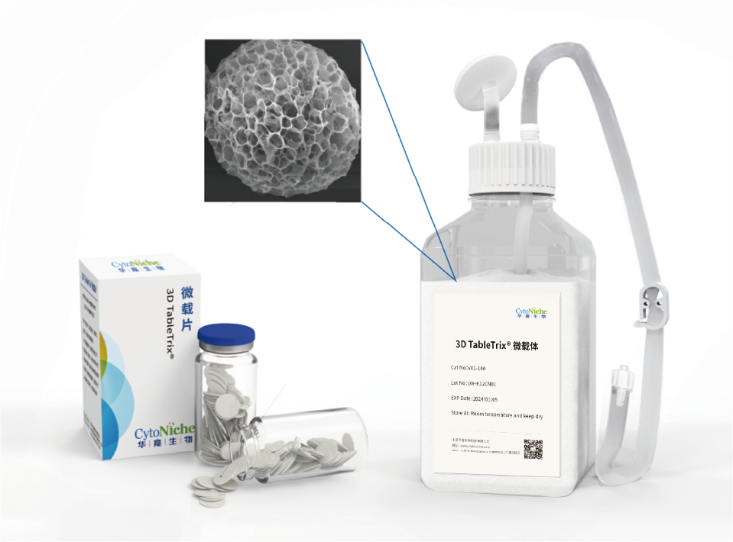
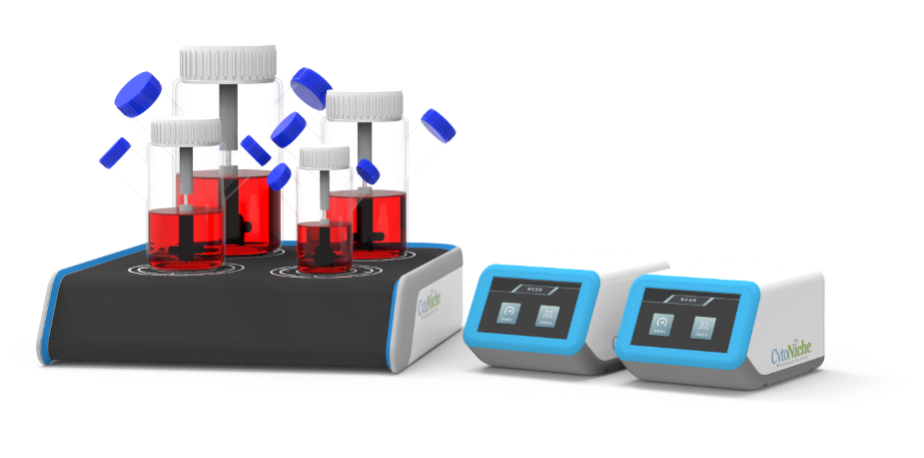
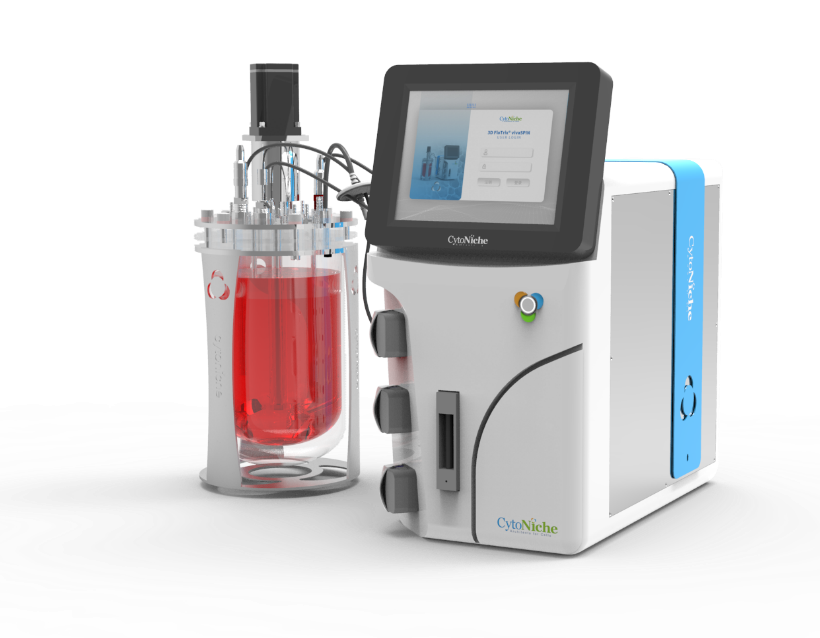
3D TableTrix® Microcarriers 3D FloTrix® miniSPIN Bioreactor 3D FloTrix® vivaSPIN Automated Bioreactor
Table 1. MDCK cell microcarrier settling, solution exchange and stirring design
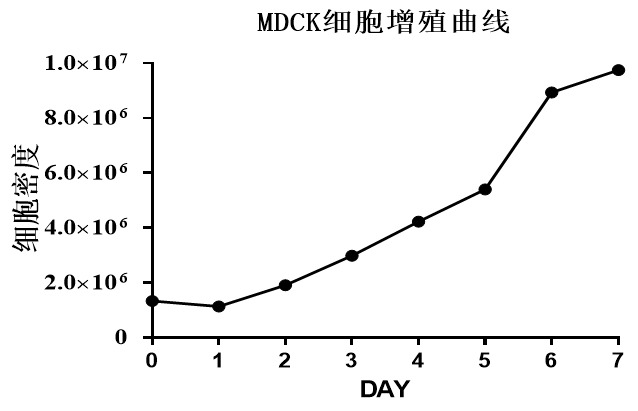

MDCK cell proliferation curve
[Continuous Scale-Up Passage Process]
Microcarriers: 3D TableTrix® Microcarriers (V-Series)
Reactor: 125 mL Gas-permeable Built-in Impeller Culture Flask (SF125)
Scheme of continuous passage: Inoculate at a cell density of 500,000/mL, proliferate 5 folds or more within 3 days, and conduct passage at 1:5.
Method: Wash the original bottle with PBS three times to remove the residual serum. After adding recombinant trypsin to digest for 10-30 min, the cells fall off the carrier (Figure 6), and then use complete medium to terminate the digestion. Pump the corresponding amounts of cells and microcarriers into the next-stage reactor for scale-up culture (Figure 7).

MDCK-D3 digested for 30 min in original bottle with recombinant trypsin
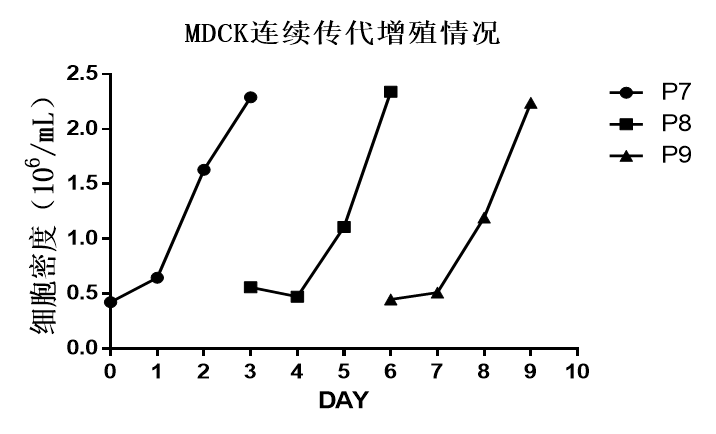
Proliferation of MDCK continuous passage
[Conclusion]
3D TableTrix® Microcarriers (V series) enable MDCK cells to be cultured in suspension in a bioreactor and to be amplified by passaging, up to a cell density of 10 million cells/mL. Overall, the unique advantages of using 3D TableTrix® Microcarriers (V-Series) in conjunction with 3D FloTrix® vivaSPIN automated bioreactor enable the desired cell volume to be harvested in a short period of time, which greatly satisfies the needs of scientific research and production, and brings new impetus to vaccine companies.
References:
[1] FENG, S.-Z., JIAO, P.-R., QI, W.-B., et al. Development and strategies of cell-culture technology for influenza vaccine[J]. Applied Microbiology and Biotechnology,2011,89(4):893-902. DOI:10.1007/s00253-010-2973-9.
[2] GREGERSEN JP, SCHMITT HJ, TRUSHEIM H, et al. Safety of MDCK cell culture-based influenza vaccines. [J]. Future microbiology,2011,6(2):143-152. DOI:10.2217/fmb.10.161.
[3] Zhao Caihong, Wang Meihao, Li Ziliang, Jin Dongwu, Ma Hua, Ma Zhongren, Qiao Zilin, Chen Hong, Zhang Jiayou, Wang Jiamin. Establishment of serum-free suspension culture MDCK cell line and high-density culture in bioreactor [J]. China Biological Products Journal of Science, 2021, 34(11): 1362-1369.DOI: 10.13200/j.cnki.cjb.003482.
[CytoNiche]
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
