CytoNiche Made it into the First KPMG China 50 Enterprises of Biotech Innovation
- Categories:Company News
- Author:
- Origin:
- Time of issue:2022-06-17
- Views:0
(Summary description)HostedbyKPMGChinaandjointlyorganizedbyChinaMerchantsSecuritiesCo.,Ltd.,theawardingceremonyofthefirstKPMGChina50EnterprisesofBiotechInnovationwassuccessfullyheldonJune15,2022.BeijingCytoNicheBiotechnologyCo.,Ltd.successfullymadeitintothelist! ClickthepicturetoreadtheoriginaltextAimingtoselectenterprisesofbiotechinnovationwithgreatpotentialandjointlypromotethehigh-qualityandhealthydevelopmentofChina'sbiotechinnovationindustry,overtenauthoritativeexpertswereinvitedasjudgestoevaluateenterprisesinthesixfieldsofbiopharmaceutical,celltherapy,genetherapy,invitrodiagnosis,medicaldevicesandpharmaceuticaldevelopmentaccordingtofivecoreevaluationdimensions. 【AboutCytoNiche】Adheringtothephilosophyofcontributingtothebiomedicalindustry,CytoNiche,aspiringtosetoffaneweraofcellindustrializationdevelopment,hascreatedanoriginal3Dcell"intelligentmanufacturing"platformthroughtransformationoforiginalscientificresearchachievements,thusgainingthefavorandrecognitionoftheindustryandsuccessfullymakingitintothelist.Biotechnologyisonanacceleratingcoursetobecomeanothernewleadingindustryfollowingtheinformationindustry.Itwillprofoundlychangethemodeofeconomicdevelopmentandlifeofhumansociety.Conformingtotheguidanceandcallofthestatetobuildthecorecompetitivenessinscienceandtechnologyandindustrialadvantage,CytoNicheforgesaheadwithinnovationtosecureitsstrategicadvantageinbiotechnology. [AboutCytoNiche]WithcoretechnologiestranslatedfromTsinghuaUniversity,BeijingCytoNicheBiotechnologyCo.,Ltd.waslaunchedbyProfessorDuYanananditsresearchteamattheSchoolofMedicineofTsinghuaUniversity. Cytoniche focusesoncreatinganoriginal3Dcell"intelligentmanufacturing"platform,andprovidesantotal solutionforthelarge-scalecustomizedamplificationprocessofcellsbasedon3Dmicrocarriers.ThetwopharmaceuticalexcipientproductsdevelopedbyCytoNicheforcelltherapy-GelatinMicrocarriersandGelatinMicrocarrierTablets(Cell)-havereceivedqualityandsafetyevaluationsbytheNationalInstitutesforFoodandDrugControl,andhaveobtained2qualificationsforpharmaceuticalexcipientsfromtheNationalMedicalProductsAdministration(CDEapprovalregistrationnumber:F20210000003,F20200000496).Thelatterhasalsoobtainedthe DrugMasterFiles(DMF)qualificationforpharmaceuticalexcipientsfromFoodandDrugAssociation(FDADMF:35481).TheproductsandservicesofCytonichecanbewidelyusedintheupstreamprocessdevelopmentofgeneandcelltherapy,extracellularvesicles,vaccinesandproteinproducts.Atthesametime,italsohasbroadapplicationprospectsinthefieldsofregenerativemedicine,organoidsandfoodtechnology(cellculturedmeat,etc.).Uptodate,thecompanyhasaR&Dand transformation platform of5000squaremeters, aGMPcertifiedmanufacturingplatformof4000squaremeters, andanew1200Lmicrocarrierproductionlinehasbeen built.Therelevanttechnologieshavebeengrantedmorethan100 patents,andtherehasbeenmorethan30articlespublishedininternationaljournals.
CytoNiche Made it into the First KPMG China 50 Enterprises of Biotech Innovation
(Summary description)HostedbyKPMGChinaandjointlyorganizedbyChinaMerchantsSecuritiesCo.,Ltd.,theawardingceremonyofthefirstKPMGChina50EnterprisesofBiotechInnovationwassuccessfullyheldonJune15,2022.BeijingCytoNicheBiotechnologyCo.,Ltd.successfullymadeitintothelist! ClickthepicturetoreadtheoriginaltextAimingtoselectenterprisesofbiotechinnovationwithgreatpotentialandjointlypromotethehigh-qualityandhealthydevelopmentofChina'sbiotechinnovationindustry,overtenauthoritativeexpertswereinvitedasjudgestoevaluateenterprisesinthesixfieldsofbiopharmaceutical,celltherapy,genetherapy,invitrodiagnosis,medicaldevicesandpharmaceuticaldevelopmentaccordingtofivecoreevaluationdimensions. 【AboutCytoNiche】Adheringtothephilosophyofcontributingtothebiomedicalindustry,CytoNiche,aspiringtosetoffaneweraofcellindustrializationdevelopment,hascreatedanoriginal3Dcell"intelligentmanufacturing"platformthroughtransformationoforiginalscientificresearchachievements,thusgainingthefavorandrecognitionoftheindustryandsuccessfullymakingitintothelist.Biotechnologyisonanacceleratingcoursetobecomeanothernewleadingindustryfollowingtheinformationindustry.Itwillprofoundlychangethemodeofeconomicdevelopmentandlifeofhumansociety.Conformingtotheguidanceandcallofthestatetobuildthecorecompetitivenessinscienceandtechnologyandindustrialadvantage,CytoNicheforgesaheadwithinnovationtosecureitsstrategicadvantageinbiotechnology. [AboutCytoNiche]WithcoretechnologiestranslatedfromTsinghuaUniversity,BeijingCytoNicheBiotechnologyCo.,Ltd.waslaunchedbyProfessorDuYanananditsresearchteamattheSchoolofMedicineofTsinghuaUniversity. Cytoniche focusesoncreatinganoriginal3Dcell"intelligentmanufacturing"platform,andprovidesantotal solutionforthelarge-scalecustomizedamplificationprocessofcellsbasedon3Dmicrocarriers.ThetwopharmaceuticalexcipientproductsdevelopedbyCytoNicheforcelltherapy-GelatinMicrocarriersandGelatinMicrocarrierTablets(Cell)-havereceivedqualityandsafetyevaluationsbytheNationalInstitutesforFoodandDrugControl,andhaveobtained2qualificationsforpharmaceuticalexcipientsfromtheNationalMedicalProductsAdministration(CDEapprovalregistrationnumber:F20210000003,F20200000496).Thelatterhasalsoobtainedthe DrugMasterFiles(DMF)qualificationforpharmaceuticalexcipientsfromFoodandDrugAssociation(FDADMF:35481).TheproductsandservicesofCytonichecanbewidelyusedintheupstreamprocessdevelopmentofgeneandcelltherapy,extracellularvesicles,vaccinesandproteinproducts.Atthesametime,italsohasbroadapplicationprospectsinthefieldsofregenerativemedicine,organoidsandfoodtechnology(cellculturedmeat,etc.).Uptodate,thecompanyhasaR&Dand transformation platform of5000squaremeters, aGMPcertifiedmanufacturingplatformof4000squaremeters, andanew1200Lmicrocarrierproductionlinehasbeen built.Therelevanttechnologieshavebeengrantedmorethan100 patents,andtherehasbeenmorethan30articlespublishedininternationaljournals.
- Categories:Company News
- Author:
- Origin:
- Time of issue:2022-06-17
- Views:0

Hosted by KPMG China and jointly organized by China Merchants Securities Co., Ltd., the awarding ceremony of the first KPMG China 50 Enterprises of Biotech Innovation was successfully held on June 15, 2022. Beijing CytoNiche Biotechnology Co., Ltd. successfully made it into the list!
Click the picture to read the original text
Aiming to select enterprises of biotech innovation with great potential and jointly promote the high-quality and healthy development of China's biotech innovation industry, over ten authoritative experts were invited as judges to evaluate enterprises in the six fields of biopharmaceutical, cell therapy, gene therapy, in vitro diagnosis, medical devices and pharmaceutical development according to five core evaluation dimensions.
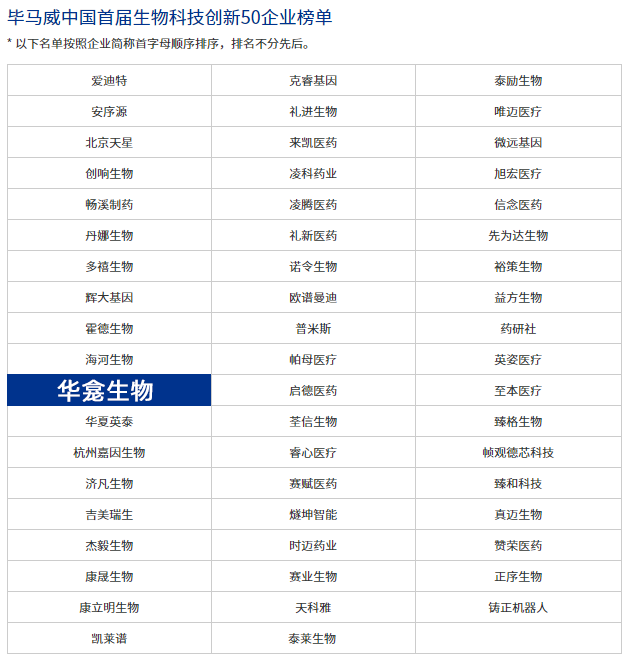
【About CytoNiche】
Adhering to the philosophy of contributing to the biomedical industry, CytoNiche, aspiring to set off a new era of cell industrialization development, has created an original 3D cell "intelligent manufacturing" platform through transformation of original scientific research achievements, thus gaining the favor and recognition of the industry and successfully making it into the list.
Biotechnology is on an accelerating course to become another new leading industry following the information industry. It will profoundly change the mode of economic development and life of human society. Conforming to the guidance and call of the state to build the core competitiveness in science and technology and industrial advantage, CytoNiche forges ahead with innovation to secure its strategic advantage in biotechnology.

[About CytoNiche]
With core technologies translated from Tsinghua University, Beijing CytoNiche Biotechnology Co., Ltd. was launched by Professor Du Yanan and its research team at the School of Medicine of Tsinghua University.
Cytoniche focuses on creating an original 3D cell "intelligent manufacturing" platform, and provides an total solution for the large-scale customized amplification process of cells based on 3D microcarriers.
The two pharmaceutical excipient products developed by CytoNiche for cell therapy - Gelatin Microcarriers and Gelatin Microcarrier Tablets (Cell) - have received quality and safety evaluations by the National Institutes for Food and Drug Control , and have obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496).The latter has also obtained the Drug Master Files (DMF) qualification for pharmaceutical excipients from Food and Drug Association (FDA DMF: 35481).
The products and services of Cytoniche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines and protein products. At the same time, it also has broad application prospects in the fields of regenerative medicine, organoids and food technology (cell cultured meat, etc.).
Up to date, the company has a R&D and transformation platform of 5000 square meters, a GMP certified manufacturing platform of 4000 square meters, and a new 1200L microcarrier production line has been built.
The relevant technologies have been granted more than 100 patents, and there has been more than 30 articles published in international journals.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
