Overview of the Large-scale Expansion Process of Human Mesenchymal Stem Cell 3D Microcarriers
- Categories:Company News
- Author:
- Origin:
- Time of issue:2022-05-24
- Views:0
(Summary description)【ResearchBackground]】Stemcellsrefertoatypeofcellpopulationwithhighself-renewalanddifferentiationpotential.Underspecificinductionconditions,theycandifferentiateintovariousfunctionalcellpopulationstofor
Overview of the Large-scale Expansion Process of Human Mesenchymal Stem Cell 3D Microcarriers
(Summary description)【ResearchBackground]】Stemcellsrefertoatypeofcellpopulationwithhighself-renewalanddifferentiationpotential.Underspecificinductionconditions,theycandifferentiateintovariousfunctionalcellpopulationstofor
- Categories:Company News
- Author:
- Origin:
- Time of issue:2022-05-24
- Views:0
【Research Background]】
Stem cells refer to a type of cell population with high self-renewal and differentiation potential. Under specific induction conditions, they can differentiate into various functional cell populations to form corresponding tissues or organs. Stem cells can subvert conventional treatment methods and have great potential in the treatment of many major diseases. Using stem cell transplantation, it has been possible to treat a variety of major diseases that cannot be solved by conventional medical methods. As of April, 2022, 26 stem cell products in our country have been approved for clinical trials through new drug applications. The most widely used of these products are mesenchymal stem cells derived from different tissues.
【Culture Method】
At present, the culture modes of stem cells include two-dimensional (traditional) culture and three-dimensional culture.
In the process of clinical transformation and application of mesenchymal stem cells (hMSCs), the process of culture, passage and expansion of stem cells is an important part of CMC (chemistry, manufacturing, controls) for cell therapy products. Stem cell culture modes include two-dimensional (traditional) culture and three-dimensional culture. In the previous article, "An Overview of the Characteristics of 2D and 3D Culture of Stem Cells", their respective process features have been introduced.
Click the picture to read the original text
At present, the production process of stem cell therapy products entering the clinical trial stage through IND basically adopts the traditional two-dimensional culture process in culture flasks, and multi-layer cell factories are used in a few cases. That is, hMSCs are directly inoculated into cell culture flasks or cell culture dishes for adherent culture to achieve the purpose of cell culture and expansion. However, these methods have disadvantages such as small available growth area for cells, large footprint of cell culture vessels, and inconsistent quality of cells harvested.

Figure: The cell factory Image source: Internet
At present, some new methods of three-dimensional expansion and culture that can significantly improve the expansion efficiency of stem cells have been developed, such as hollow fiber culture system, microcarrier-based suspension culture technology, and porous biomaterial culture technology. Among them, the microcarrier suspension culture technology is widely favored because it is convenient for large-scale culture and cell collection. However, most of the commercial microcarriers currently on the market are aimed at harvesting cell expression products, which cannot meet the purpose of cell therapy companies to harvest stable quality cells that meet the needs of clinical applications. Existing cell therapy companies must not only select suitable microcarriers to harvest adequate number of stem cells required by stem cell industrialization, but also ensure the critical quality attributes of stem cells. Therefore, the cell expansion process in the traditional microcarrier application field cannot be simply applied. The automated stem cell 3D expansion and culture system, developed based on the dissolvable microcarriers by CytoNiche, can meet the requirements of large-scale expansion and closed harvesting of hMSCs.
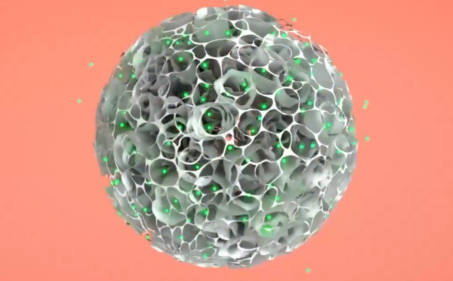
Figure: Cell Culture via 3D TableTrix® Microcarriers (Porous Microcarriers)
【3D Culture Mode of CytoNiche】
3D Culture and Expansion process of MSC in CytoNiche:
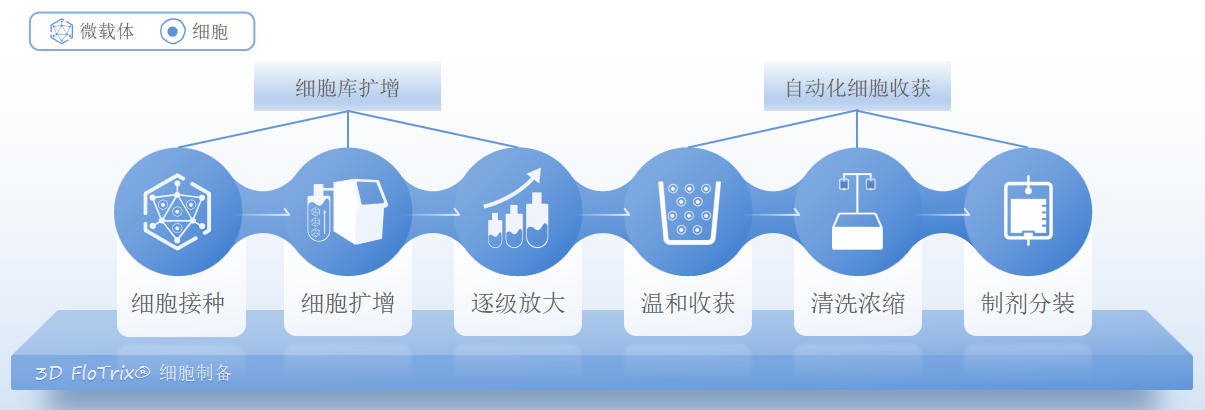
The primary harvested cells are expanded to establish a seed bank by the traditional 2D process, and the influencing factors such as cell inoculation volume, stirring speed, and dissolved oxygen are optimized according to the cell characteristics. Different 3D expansion systems: 500 mL, 5 L, 10 L-15 L, are used to achieve stepwise scale-up of cells.
Using the dissolvable properties of the microcarriers, when the cells reach the expected number on the microcarriers, they are lysed in the original flask in combination with the specific microcarrier lysate to achieve gentle harvesting of cells. The number of cells that can be harvested in a single batch is about 100 times that of the T175 cell culture flask. Meanwhile, the quality standards of cell therapy companies can be met and the uniformity of cells can be guaranteed.
【Case Sharing】
• Cells inoculated UCMSCs
• Microcarriers 3D TableTrix® Microcarrier Tablet
• Culture vessel vivaSPIN VS05
• Culture system 4.5 L
1.1 Cell Proliferation and Metabolism

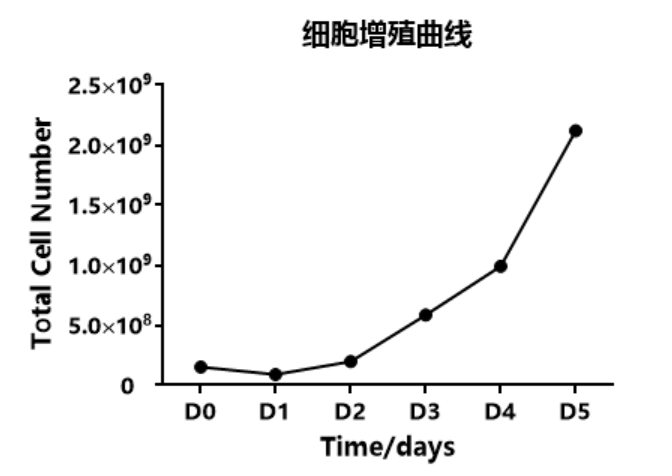
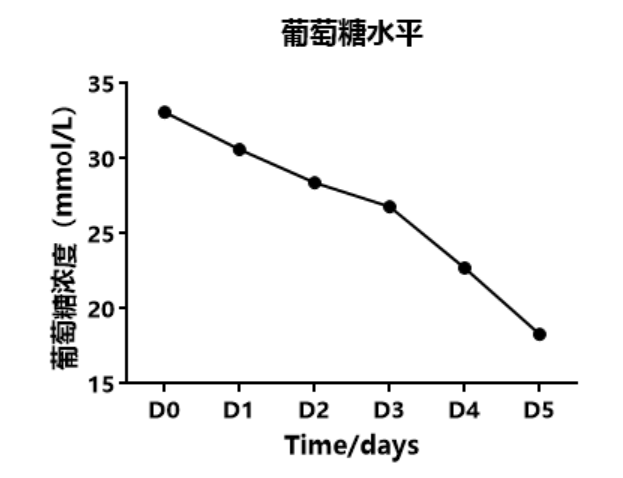
Figure: Cell proliferation curve and metabolism curve
1.2 Identification
1.21 Cell Morphology
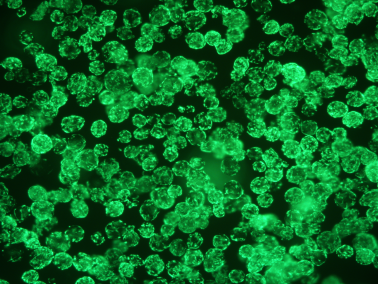
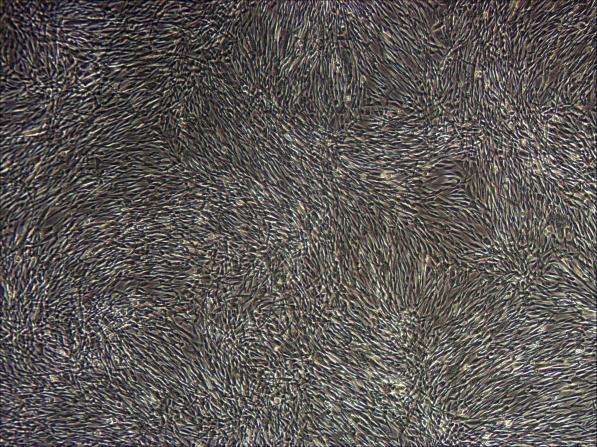
Figure: 3D culture state Figure: Two-dimensional attachment state of 3D harvested cells
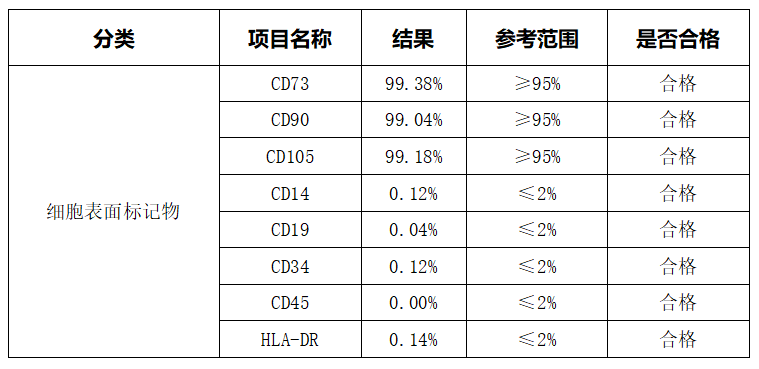
1.22 Cell Surface Markers
1.23 STR Profile

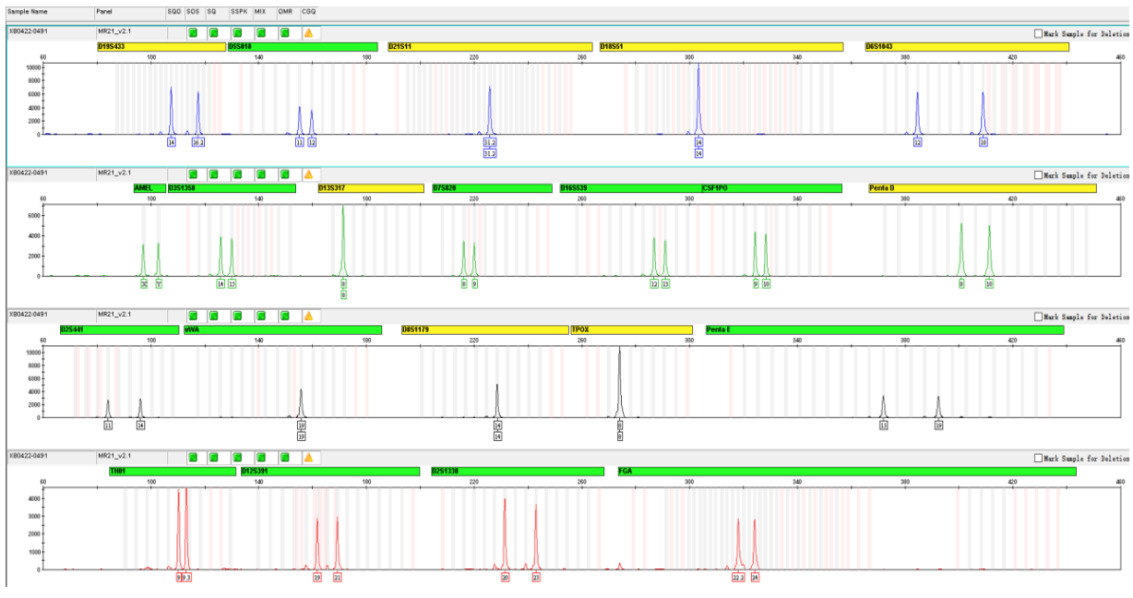
Figure: STR typing profile of 3D harvested cells
1.3 Microbial Test
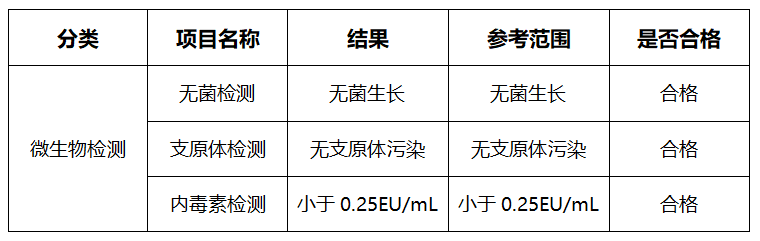
1.4 Monitoring of Tumorigenicity
1.41 Soft Agar Assay
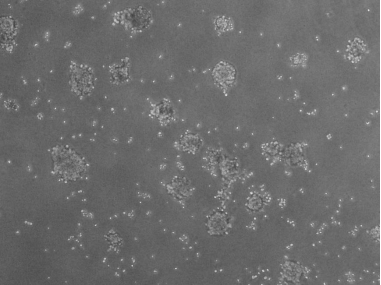

Figure: Positive Figure: 3D harvested cells
1.42 Detection of Telomerase Activity
(Blue is the TERT curve, light green is the internal reference GAPDH curve)
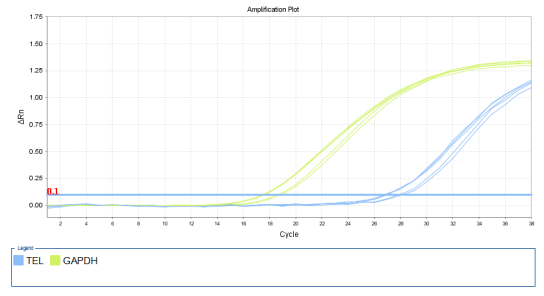
Figure: Amplification curve of positive cell 293T
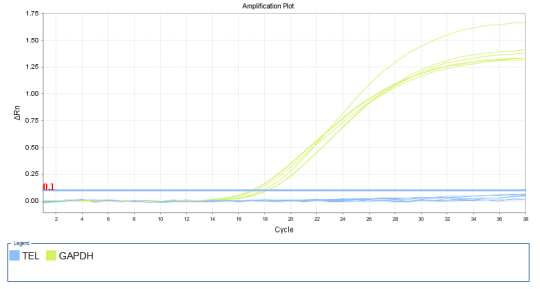
Figure: Amplification curve of negative cell MRC-5
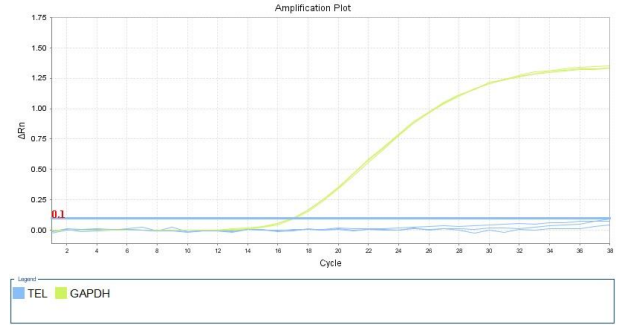
Figure: Amplification curve of 3D harvested cells
1.5 Tri-lineage Differentiation
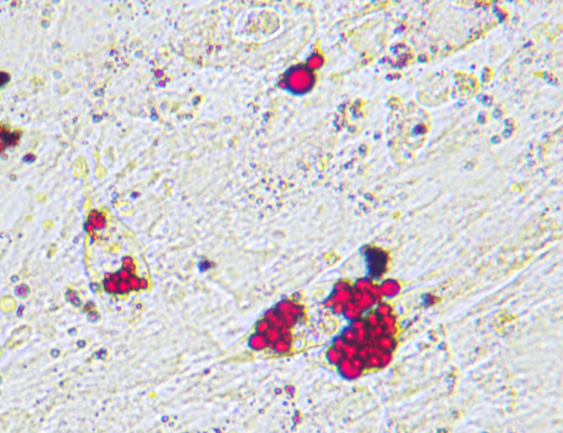
Figure: Lipogenesis
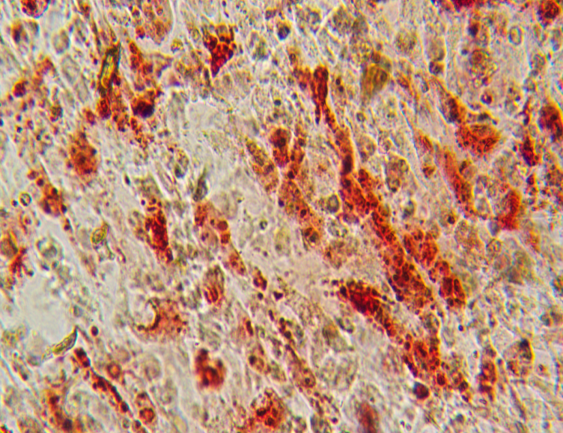
Figure: Osteogenesis
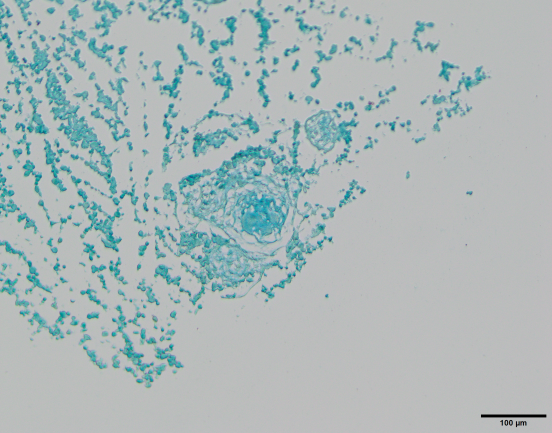
Figure: Cartilage
【Conclusion】
Fully automatic, closed cell processing system is the process development trend in the field of CGT
CytoNiche's large-scale and automated cell manufacturing system has the following advantages:
1. The closed automated system ensures aseptic manufacturing of cells;
2. It is easy to expand the production scale and facilitate quality control;
3. It can significantly reduce production costs.
On this basis, CytoNiche provides overall solutions for the 3D microcarrier-based customized cell expansion process, which meets the quality and regulatory requirements of cell therapy final products, and facilitates the commercial production of cell therapy products.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP




 京公网安备 11010802037749号
京公网安备 11010802037749号
