CytoNiche and EurekaBio Reached a Strategic Cooperation to Jointly Build a Closed and Large-Scale Stem Cell "Intelligent" Manufacturing Platform
- Categories:Company News
- Author:
- Origin:
- Time of issue:2021-09-10
- Views:0
(Summary description)On September 10, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as CytoNiche) and Shenzhen Eureka biotechnology Co. Ltd. (hereinafter referred to as EurekaBio) signed a strategic cooperation agreement.
Dr. Liu Wei (CEO) and Mr. Wang Zhiqian (Director of Marketing and Sales Center) from CytoNiche, were joined by Mr. Liu Bin (COO), Dr. Xue Bofu, (CSO), and Mr. Huang Mouzhen, Manager of Quality Regulation Department from EurekaBio, to attend the signing ceremony.
CytoNiche and EurekaBio Reached a Strategic Cooperation to Jointly Build a Closed and Large-Scale Stem Cell "Intelligent" Manufacturing Platform
(Summary description)On September 10, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as CytoNiche) and Shenzhen Eureka biotechnology Co. Ltd. (hereinafter referred to as EurekaBio) signed a strategic cooperation agreement.
Dr. Liu Wei (CEO) and Mr. Wang Zhiqian (Director of Marketing and Sales Center) from CytoNiche, were joined by Mr. Liu Bin (COO), Dr. Xue Bofu, (CSO), and Mr. Huang Mouzhen, Manager of Quality Regulation Department from EurekaBio, to attend the signing ceremony.
- Categories:Company News
- Author:
- Origin:
- Time of issue:2021-09-10
- Views:0
On September 10, Beijing CytoNiche Biotechnology Co., Ltd. (hereinafter referred to as CytoNiche) and Shenzhen Eureka biotechnology Co. Ltd. (hereinafter referred to as EurekaBio) signed a strategic cooperation agreement.
Dr. Liu Wei (CEO) and Mr. Wang Zhiqian (Director of Marketing and Sales Center) from CytoNiche, were joined by Mr. Liu Bin (COO), Dr. Xue Bofu, (CSO), and Mr. Huang Mouzhen, Manager of Quality Regulation Department from EurekaBio, to attend the signing ceremony.

Based on the core technology of 3D TableTrix® Microcarrier Tablet, CytoNiche has developed 3D FloTrix® products for the automated and large-scale cell culture process, addressing the technical difficulties in the cell industrialization industry including high cost, low yield per batch, quality controls, and the standardized operations, so as to realize the "intelligent manufacturing" of large-scale and automated production of cell therapy products.
EurekaBio, a high-tech enterprise devoted to the research and development of critical technologies and process in the field of gene therapy and cell therapy, has developed a closed and automated CellSep PRO® cell processing system, which enabled eclosed and automated production of immune cell therapy products.
Relying on their respective competitive advantages in the industry and products, the two parties jointly developed the VivaPREP customized device for large-scale cleaning, harvest, and aliquotation of cells, which can seamless connect with CytoNiche's 3D FloTrix® large-scale cell process system to meet the requirements of fully closed cleaning and harvesting after the large-scale production of cell formulations, thus truly realizing the continuous closed large-scale production and preparation process system of cell formulations, and meeting the latest regulatory requirements for the cell drug development.
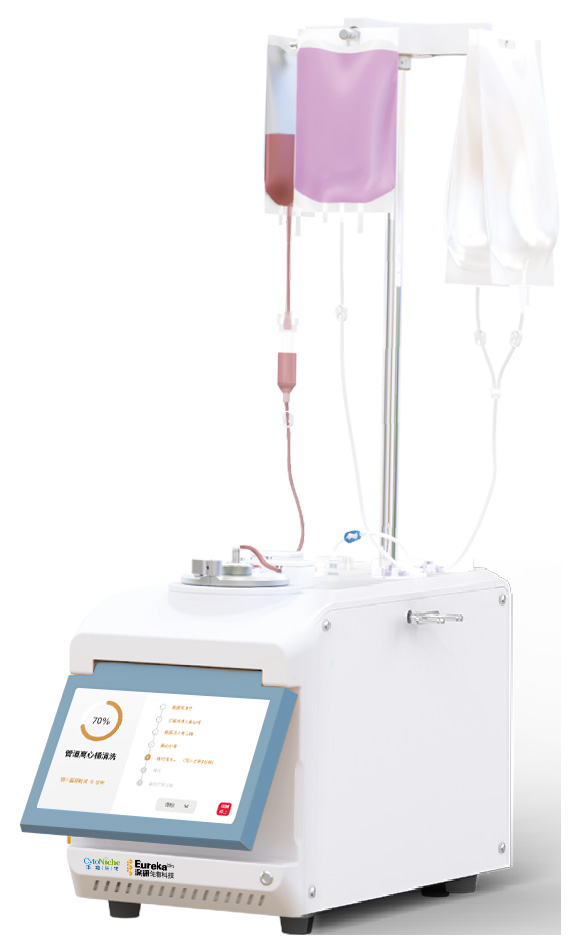
Demo of CellSep PRO customized VivaPREP cell harvesting system
The strategic cooperation between the two companies will enable their joint effots to dedicate to the application of stem cells, facilitating the cell product industrialization, and realizing the grand vision of changing the field of biomedicine with scientific and technological innovation.

Dr. Liu Wei, CEO of CytoNiche, said:
Since the "14th Five-Year Plan", China's biological cell field has embraced a historical opportunity for innovation and development. With the support of policies, CytoNiche has also made breakthroughs in the fields such as 3D cell large-scale production and preparation as well as regenerative medicine.
n this strategic cooperation with EurekaBio, we will leverage our competitive advantages in the upstream in the industry and technical expertise in the end-to-end industry solution. Together with the advantages of fully closed and automated cleaning and harvesting system for cell formulations from EurekaBio, we hope to help customers overcome the bottleneck, realizing the high-quality and large-scale preparation of cells and cell products, and meeting the needs of drug filings.
Mr. Liu Bin, COO of EurekaBio, said:
EurekaBio is committed to the research and development of critical technology and process development in the field of cell therapy and gene therapy, and has abundant experience in the development of cell production equipment.
The signing of the strategic cooperation agreement marked the official partnership between CytoNiche and EurekaBio Bio.
Based on the CellSep PRO fully closed and automated cell processing system, together with CytoNiche's leading 3D culture technologies, the two parties will develop a customized VivaPREP automated cell formulation cleaning, harvesting and aliquoting device that is applicable to the 3D FloTrix® production process, integrating the advantages of both parties to carry out in-depth strategic cooperation in business channels, market development, and product innovation, thus jointly expanding the market in the field of stem cell applications.
We believe that our cooperation will enable both parties to make a difference in the stem cell market.
[About CytoNiche]
Beijing CytoNiche Biotechnology Co., Ltd. was incubated by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation.The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University.Its proprietary 3D FloTrix® series of products and supporting processes provide an overall solution for the large-scale production and preparation of cell therapy products, and they are also proprietary innovative injection-type pharmaceutical excipient products that can be loaded with stem cells.
The two pharmaceutical excipient products developed by CytoNiche for the production and preparation of cell formulations, Gelatin Microcarrier and Gelatin Microcarrier Tablet for Cells, have passed the test for quality evaluation and safety evaluation of the National Institutes for Food and Drug Control, and have obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20210000496).Besides, the latter one has obtained the DMF qualification for pharmaceutical excipients from US FDA (DMF: 35481).
The company has a research and development center of 1500 square meters and a GMP certified manufacturing platform of 2000 square meters.Over 50 patents have been granted owing to the relevant technologies, and more than 30 articles about the technologies in international journals have been published.
[About EurekaBio]
Eurekabio is a scientific and technological enterprise focusing on the research and development of core technologies and processes in the fields of gene therapy and cell therapy.The company is committed to discovering innovative ways in the field of life science through the interdisciplinary disciplines of computer science, electronic engineering, nanomaterials, chemical engineering and life sciences. We are devoted to the rapid evolution in life science and technology,
and to gradually solve a series of critical technical challenges in the fields of gene therapy, cell therapy, life sciences, and pharmaceutical research and development.
Based on the combination of automation technology and biotechnology, Eurekabio has developed the CellSep PRO® automated cell processing system and its complimentary consumables, which can automatically complete the core processes such as cell separation, concentration, dilution, washing, and replacement in a closed system, thus facilitating cell therapy technologies in upgrading from manual preparation to standardized production. And this is the first domestically produced cell processing system.
In order to tackle the challenge of lentiviral vector production, Eurekabio has innovatively developed the EuLV® system and related scale-up processes for large-scale production of lentiviral vectors based on stable production of cell lines, which solves the technical bottleneck of large-scale production of lentiviral vectors, thus greatly improving the production efficiency and product consistency of lentiviral vectors, and greatly reducing the production cost.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
