[Carrier Selection] in the Cell Culture Process
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-03-02
- Views:790
(Summary description)CytoNiche is able to provide a complete set of solutions based on different cells on microcarriers from inoculation and culture to system amplification and harvesting, which has been widely applied in
[Carrier Selection] in the Cell Culture Process
(Summary description)CytoNiche is able to provide a complete set of solutions based on different cells on microcarriers from inoculation and culture to system amplification and harvesting, which has been widely applied in
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2023-03-02
- Views:790
[Microcarriers]
Here, we will provide more details on microcarrier cell culture from the following aspects for your reference.
[Carrier structure and material]
① Structure:
Carriers are usually available in solid microcarriers (infarctate microcarriers and porous microcarriers) and liquid microcarriers.
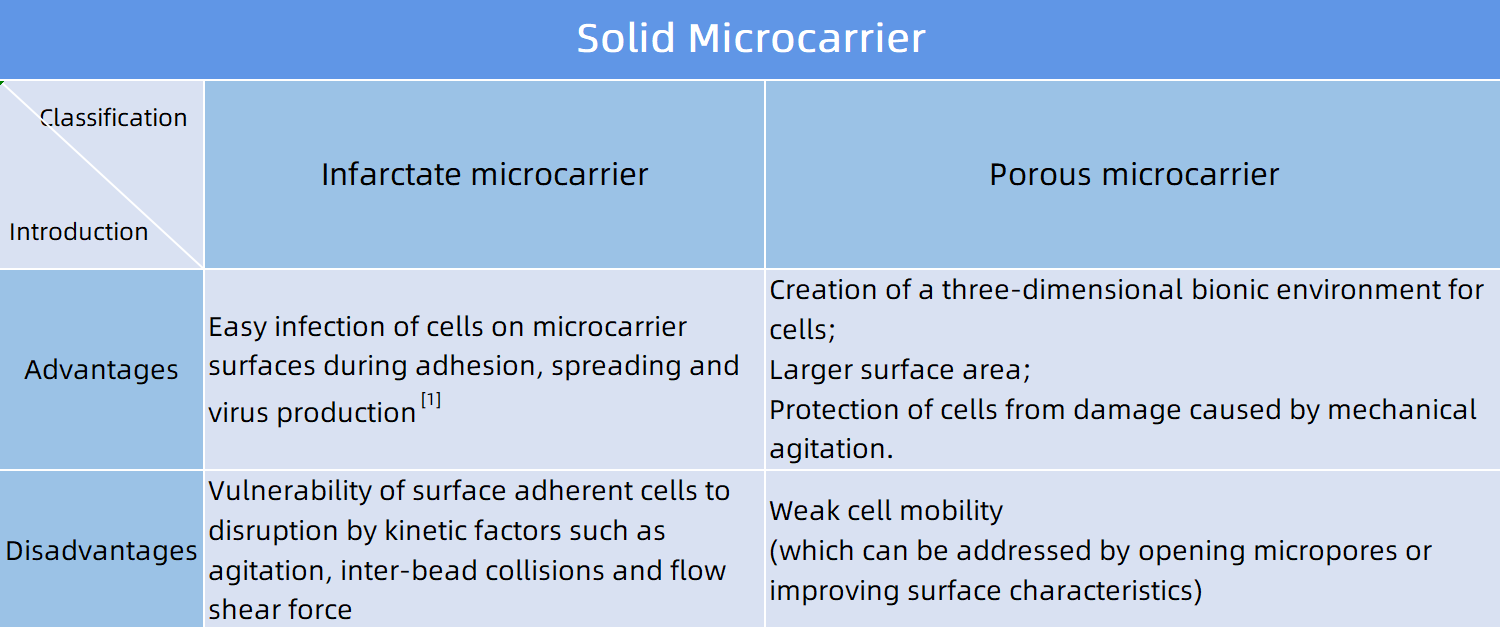
Figure 1: Classification of solid microcarriers and analysis of their advantages and disadvantages (click to view larger image)
② Materials:
The materials of microcarriers are also a key factor in cell culture, because they have physical and chemical interactions with cells, including their porosity, mechanical strength, permeability of nutrients, size, density and shape [2].
▷ Synthetic polymer materials:
For example, polystyrene microcarriers, hollow glass microcarriers, polystyrene phthalamide microcarriers;
▷ Natural polymer materials:
For example, cross-linked gelatin microcarriers, cellulose microcarriers, chitosan microcarriers, alginic acid, hyaluronic acid;
▷ Other inorganic materials:
For example, glass microcarriers.
[Adhesion, proliferation and harvesting of adhesion-dependent animal cells on microcarriers]
① Factors affecting cell adhesion by microcarriers [2]
(1) Compatibility of cells and microcarriers
The compatibility of cells with microcarriers has a close bearing on the chemical and physical properties of the substrate surface of microcarriers. Generally, cells become negatively charged as they reach physiological pH. If the microcarriers are positively charged, electrostatic attraction will speed up the cell adhesion. Conversely, if the microcarriers are negatively charged, electrostatic repulsion will make cell adhesion difficult to a large extent; however, when divalent cations are dissolved in the culture medium or adsorbed on the surface of the microcarrier as a medium, negatively charged cells can also adhere.
For conventional microcarriers commonly available on the market, they use basic substrates made of polymers (e.g., polystyrene, dextran, glass, etc.) that are electroneutral and cannot support cell adhesion. For this reason, modification of the positive charge or chemical bonding is often required to facilitate the adhesion of adherent cells with uneven surface and negative charge distribution. Instead, microcarriers of higher charge density have been developed to promote cell adhesion of weak cell lines (e.g., Cytopore 1 and 2).
▷ Chemical modification in microcarrier surface:
△ Combining chemical groups to the microcarrier surface is a common method, through which the hydrophilicity or hydrophobicity of its surface charge is modified, for example, the microcarrier surface is modified by amino, primary amine, tertiary amine and triethylamine for electrical properties or subject to carboxylation or hydroxylation, thus improving the hydrophilicity of the microcarrier;
△Proteins containing relevant cell attachment sites are introduced, such as fibronectin, collagen and laminin.
(2) Physical characteristics of the microcarrier surface (which is porous or infarctate), including morphology and roughness.
(3) Probability of cells in contact with microcarriers.
▷ Inoculation density of carriers and cells;
▷ Agitation conditions: lower agitation speed is beneficial to cell inoculation, while MSC is more suitable for inoculation in underspeed manner [2].
② Factors affecting cell proliferation on the microcarrier surface
Below are the three main factors that affect the proliferation or differentiation of cells on the microcarrier surface:
(1) In terms of cells: such as cell population, activity, state and type.
(2) In terms of microcarriers: such as biochemical properties of the microcarrier surface, adsorbed macromolecules and ions; fast cell expansion in the case of smooth microcarrier surfaces while slow cell expansion in the case of porous microcarrier surfaces; physical characteristics of the surface, such as stiffness, elastic modulus and morphology. It is reported by literatures that the rigid surface with stiffness at 34Kpa will make MSCs fusiform and differentiate into bones, the low surface stiffness (at 1Kpa) will promote the differentiation of cartilage, fat and neurons, while the medium surface stiffness will promote the differentiation into muscles [2].
(3) In terms of culture environment: such as composition of culture medium, temperature, pH, oxygen and metabolic waste, which significantly affect the growth of cells on microcarriers. Cells grow fast under optimal conditions and vice versa.
③ Harvesting of adhesion-dependent animal cells on microcarriers:
Easy harvesting of cells or cell products is a key measure of excellent microcarriers, as well as one of the key considerations for users to choose carriers.
Currently, conventional microcarriers that are commercially available mainly use non-mildly degradable polymers (e.g., polystyrene, dextran, cellulose or glass) as the basic substrates. Therefore, the adequate separation of cells from the microcarriers at the end of culture becomes a challenge for these microcarriers during the cell harvesting [2]. Especially, such microcarriers have their charge enhanced by surface modifications for the purpose of achieving a strong cell adhesion effect. This, however, requires strong enzymatic digestion and long-time consumption for cell separation, and complete digestion of the cells cannot be guaranteed, with the result that greater cell loss and cell damage may be induced.
On the other hand, non-degradable microcarriers and cells are both solid substances, which cannot be separated by conventional centrifugation alone. Additional gradient centrifugation or filtration methods are needed to remove microcarriers and to obtain cells, making the whole production process more expensive and complex, and reducing the recovery rate and activity of cells. Furthermore, there are potential safety hazards when the harvested cells are used for treatment, because residues of such microcarrier particles will lead to safety problems such as blood vessel blockage or foreign body rejection in the subsequent injection of cell products if they cannot be effectively removed.
[Selection of suitable microcarriers for different application scenarios]
In conclusion, it is clear that whether cells can adhere, grow and expand on the microcarrier surface depends on the characteristics of cells, the physical and chemical properties of microcarriers, the composition of culture medium and the culture conditions. Also, the performance of microcarriers is a key factor affecting not only cell morphology, adhesion rate, growth rate, cell density, and easy harvesting of cells or their secreted products, but also the overall production cost of cell products.
For different cell types, it is necessary not only to screen suitable microcarriers, but also to select suitable inoculation and culture conditions. However, most cell culture R&D and production enterprises have no very comprehensive understanding on each type of microcarriers and may overlook many key factors in their selections.
Currently, CytoNiche can provide a complete set of solutions based on different cells on microcarriers from inoculation and culture to system amplification and harvesting, which has been widely applied in cell therapy, virus vaccine, genetic engineering and tissue engineering.
[Examples]
① Current application of microcarriers in the bio-pharmaceutical field - cell therapy
Billions of cells and a two-dimensional platform are required for mesenchymal cells (MSCs) in clinical practice, which may pose a challenge to large-scale production and preparation.
For a scalable number of undifferentiated MSCs in cell transplantation and tissue engineering application, three-dimensional culture technology is a more reliable method compared with two-dimensional culture [2].
For example, CytoNiche can yield a 1010-level MSC preparations in a single run by using its original 3D TableTrix®W series porous biodegradable microcarriers and carrier-only lysates, combined with automated bioreactors and cell harvesting devices.
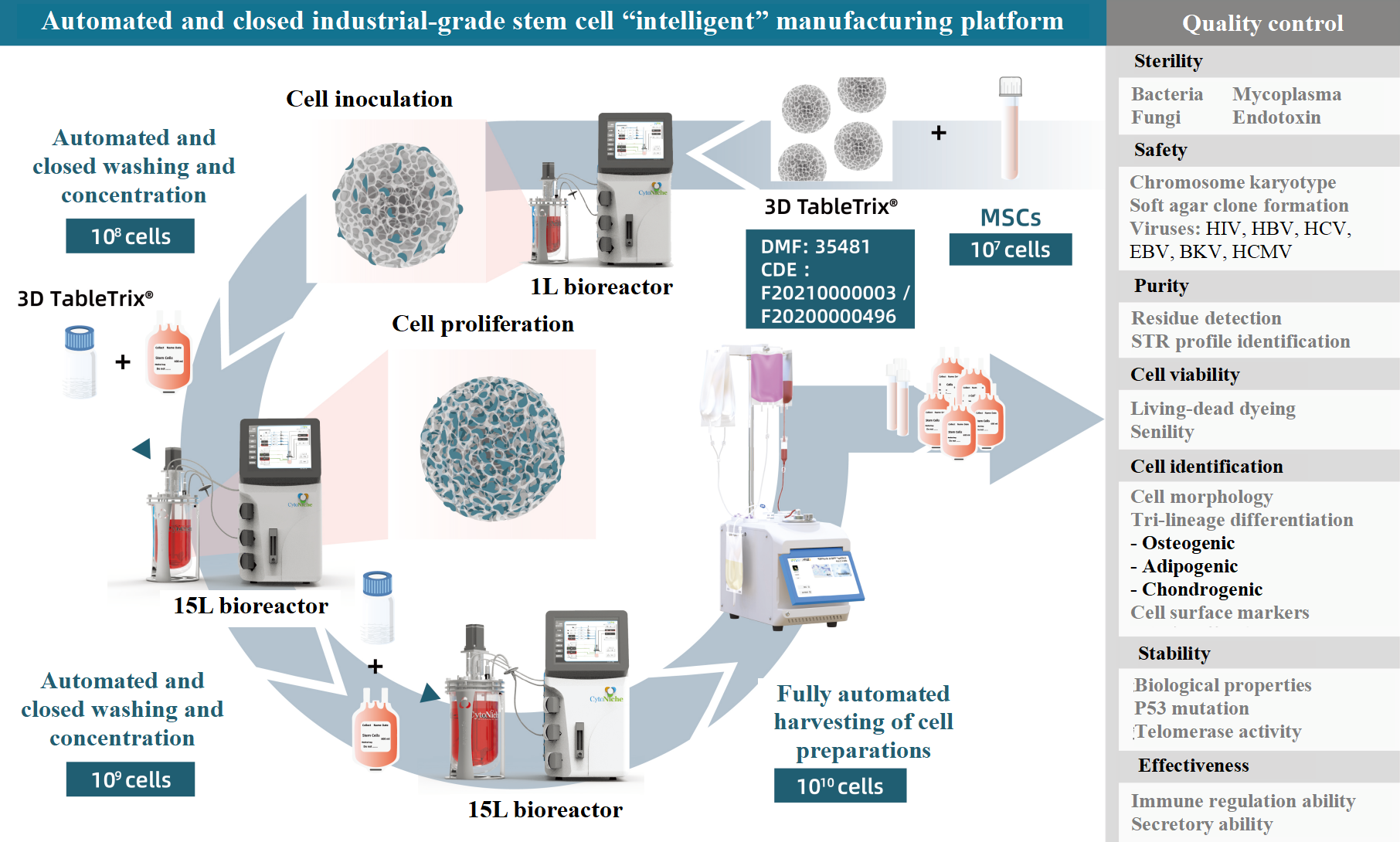
Figure 2: Production schematic diagram of automated and closed industrial-grade stem cells [4]
② Current applications of microcarriers in the bio-pharmaceutical field - virus vaccine (vero cells) [3]
It is suggested in this article that the industrialized microcarrier technology provides a powerful platform for virus production using Vero cell lines, but the platform faces some challenges in providing higher viral productivity. Compared with other animal cell lines, vero cells have lower density, generally no more than 107 cells/mL (Figure 3).
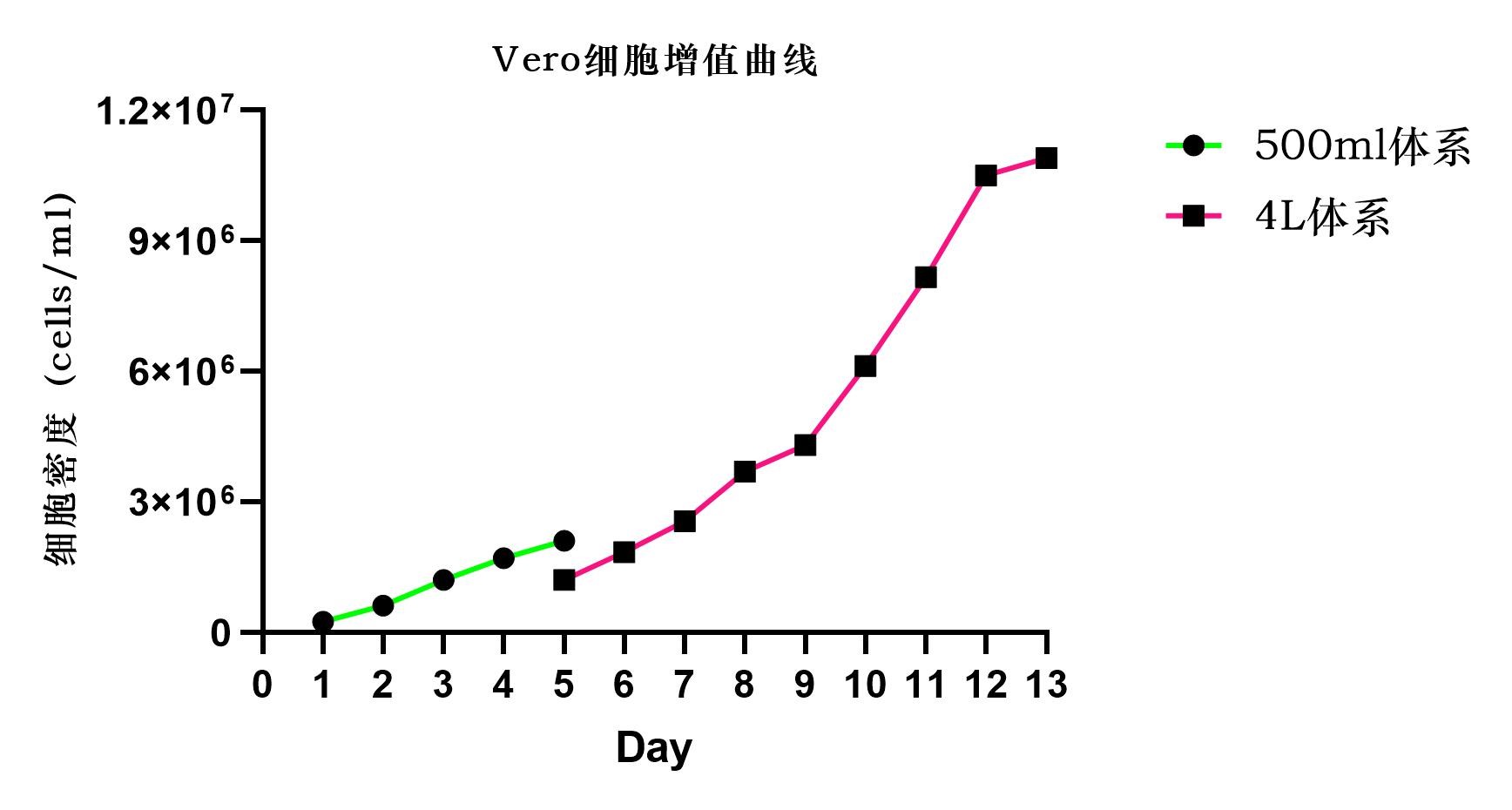
Figure 3: Vero cell proliferation curve
The following solutions are provided in this document for your reference:
(1) Development of more in-depth studies: analysis of the state of Vero cells at high cell concentration (e.g., adequate studies in metabonomics, transcriptomics and protein histology).
(2) Adoption of a more appropriate culture strategy: real-time detection of the concentration of nutrients and analytes, and timely supply and discharge [3].
In fact, CytoNiche has already achieved a high cell culture density (1×107 cells/mL) using 3D TableTrix®V series autoclavable microcarriers combined with the 3D FloTrix®vivaSPIN automated bioreactor at 4g/L-10g/L microcarrier in a one-week continuous culture. Vero cells show preferable adhesion on the microcarrier, with cell densities suitable for mass production of vaccines, and furthermore, the relevant study data has demonstrated that its efficiency of virus production is significantly higher than that of conventional microcarriers (Figure 4).
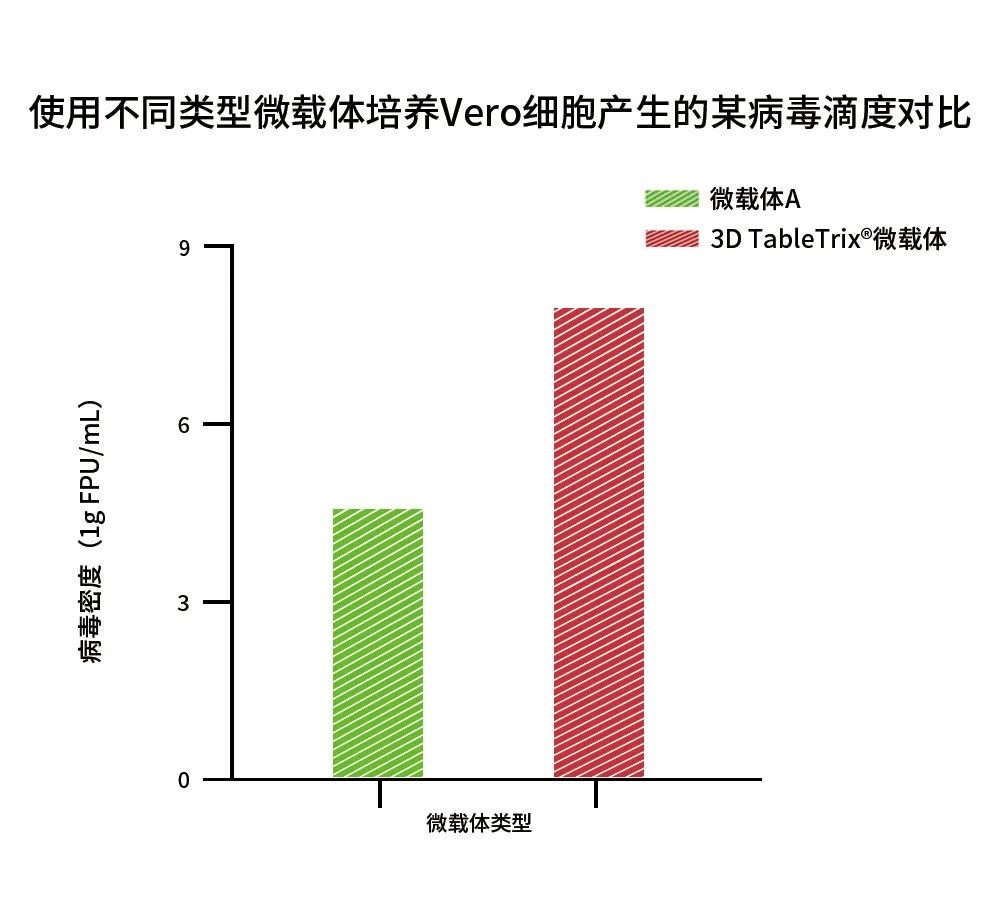
Figure 4: Comparison of a virus titer by Vero cells cultured with different types of microcarriers
[References]
[1] Berry JM, Barnabé N, Coombs KM, Butler M. Production of reovirus type-1 and type-3 from Vero cells grown on solid and macroporous microcarriers. Biotechnol Bioeng. 1999 Jan 5;62(1):12-9. doi: 10.1002/(sici)1097-0290(19990105)62:1<12::aid-bit2>3.0.co;2-g. PMID: 10099508
[2] Chen AK, Reuveny S, Oh SK. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: achievements and future direction. Biotechnol Adv. 2013 Nov 15;31(7):1032-46. doi: 10.1016/j.biotechadv.2013.03.006. Epub 2013 Mar 24. PMID: 23531528.
[3] Kiesslich S, Kamen AA. Vero cell upstream bioprocess development for the production of viral vectors and vaccines. Biotechnol Adv. 2020 Nov 15;44:107608. doi: 10.1016/j.biotechadv.2020.107608. Epub 2020 Aug 5. PMID: 32768520; PMCID: PMC7405825.
[4] Zhang Y, Na T, Zhang K, Yang Y, Xu H, Wei L, Xu L, Yan X, Liu W, Liu G, Wang B, Meng S, Du Y. GMP-grade microcarrier and automated closed industrial scale cell production platform for culture of MSCs. J Tissue Eng Regen Med. 2022 Aug 5. doi: 10.1002/term.3341. Epub ahead of print. PMID: 35929499.
【CytoNiche】
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
