[CytoNiche’s Global Footprints US & Japan] 2022 Overseas Events Feature
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-12-30
- Views:0
(Summary description)
[CytoNiche’s Global Footprints US & Japan] 2022 Overseas Events Feature
(Summary description)
- Categories:Company News
- Author:CytoNiche
- Origin:CytoNiche
- Time of issue:2022-12-30
- Views:0
[Invitation to Contributors]
As the global bio-pharmaceutical industry is flourishing, the research and application of the state of the art in the cell industry keeps growing. Beijing CytoNiche Biotechnology Co., Ltd. (“CytoNiche”), engaged in the cell industry where rapid changes are emerging and advanced ideas are shared, attaches great importance to the communication and cooperation with the overseas markets.
Moving with the international situation and answering the call of our land for a combat against the 2019 novel coronavirus in the past years, we have been in effective cooperation and communication with foreign experts and customers through online meetings in most cases to take active actions for market opportunities abroad.
As a result of the increasingly stabilized global situation and the effective control of the pandemic, we have received numerous positive signals from the overseas market, and therefore [CytoNiche’s Global Footprints] gets started from now on.
Stop 1: U.S.
① Boston · Bio Process International 2022
How could CytoNiche be absent from the largest exhibition event in the United States on the commercial production of biopharmaceuticals with exhibitors from and topics covering nearly all the upstream and downstream businesses around cell therapy?


Additionally, we shared an amazing report on site, with the focus on the 3D cell “intelligent manufacturing” system based on the pharmaceutical grade 3D TableTrix® Microcarrier Tablet (Microcarrier), which attracted great attention from the guests. This also laid an important foundation for the future insightful communication and cooperation with many companies.
② San Diego · Cell and Gene Therapy Meeting on the Mesa 2022
The meeting was held to promote the cooperation among biopharmaceutical companies engaged in CGT and brought together managements and decision makers in the industry to addressing the commercialization obstacles in the fields of cell and gene therapies from insightful perspectives.
CytoNiche’s 3D Cell Intelligent Manufacturing and Regenerative Medicine Center---3D FloTrix® Cell Technology Platform has helped a number of stem cell pharmaceutical companies in establishing fully automated stem cell drug production lines, so that they are enabled to produce and prepare stem cell drugs in a scaled, automated, standard and intelligent manner. In this way, it is conducive to significantly reducing the production cost of cell therapy drugs and bridging the gap of cell quality between batches, improving the process reproducibility and reliability. This is how the Platform works to power the integrated data traceability and sustainable monitoring, which will further facilitate the compliance of quality control with requirements of industry regulations.
Based on this, we will provide CDMO service that is substantially different from the existing CGT cell preparation process, and enable technical research, process development and GMP production services on mesenchymal stem cells (MSCs) and exosomes from different tissue sources, thus making our contributions on product transformation and clinical application of innovation achievements.




Taking this opportunity, we also introduced relevant product services and technical support that CytoNiche will provide to customers who are active players in the field of stem cell pharmaceuticals from South Korea, Australia, Sweden and other countries, unveiling the future in-depth communication and cooperation with them.
[Stop 2: Japan]
① To A General Chemical Materials Group
The group is mainly engaged in businesses related to chemicals, functional materials, chemicals for agricultural purposes, pharmaceuticals and advanced materials in Japan and abroad.
During the trip to Japan, CytoNiche had a two-hour talk with the group’s decision makers and technical experts on cooperation strategies at its headquarters, where they said that they were impressed by the original technology and developed products of CytoNiche and they were also optimistic to the 3D cell culture technology. A consensus was also reached between both sides on specific goal of future cooperation.
② To A Prominent Pharmaceutical Company
The company is mainly engaged in medicine, cosmetics and many other services in 110 countries around the world and is also devoted to the industrialization of regenerative medicine and cell therapy.


CytoNiche was invited to pay a visit to the stem cell drugs and cosmetics R&D headquarters, where the cooperation proposal with its senior management and technical leadership was and discussed. They thought highly of the overall production and preparation solutions of CytoNiche. Also, they took a sanguine view of the future development of the stem cells and regenerative medicine market and looked forward to establishing broad and deep strategic partnership with CytoNiche.

CytoNiche is acting globally, beyond these. We are trying to find opportunities and, more importantly, learn and study more cutting-edge concepts and technologies from a global perspective. The visits to different manufacturers have brought us deeper understanding of the overseas market demands and the priorities in technology development, enabling us to think more about the development of CytoNiche’s overseas operation and business strategy in the future.
In the context of globalization, we even more firmly believe that only the technological innovation is our cornerstone and driving force for sustainable development and in response to the call of the time and tide. And we are even more determined to get involved in the reform of the global pharmaceutical industry.
In the near future, CytoNiche will leave more footprints in the global market. Please stay tuned.
【CytoNiche】
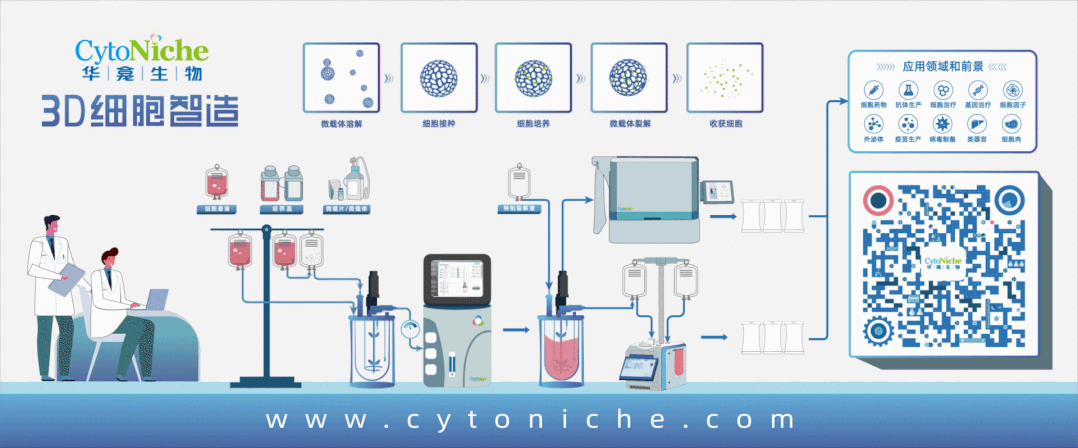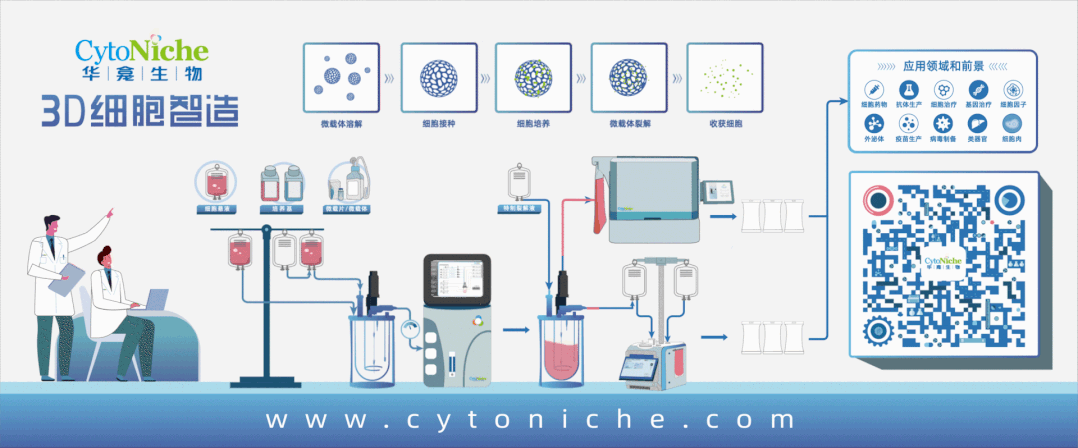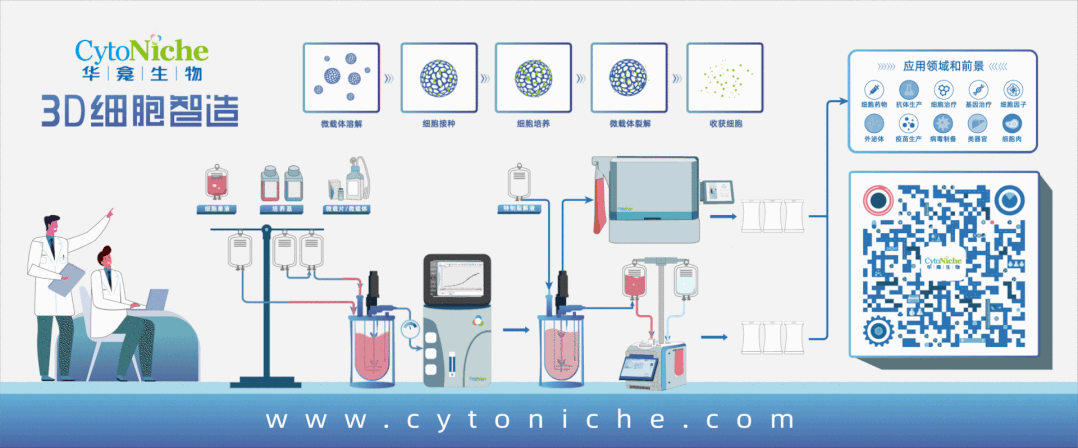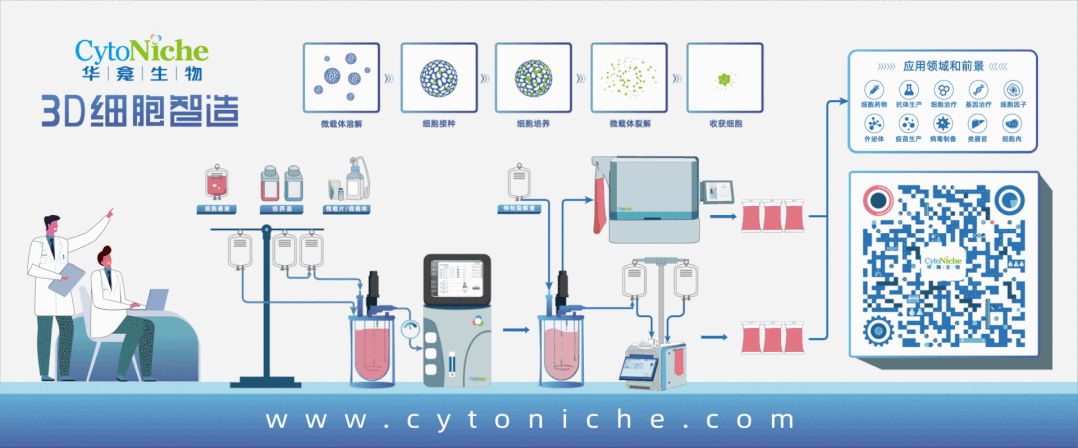
Beijing CytoNiche Biotechnology Co., Ltd. was established by the research team of Professor Du Yanan from Tsinghua University School of Medicine, and was jointly established by Tsinghua University through equity participation. The core technologies were derived from the transformation of scientific and technological achievements of Tsinghua University. CytoNiche focuses on building an original 3D cell "smart manufacturing" platform, as well as providing overall solutions for the 3D microcarrier-based customized cell amplification process.
CytoNiche's core product, 3D TableTrix® Microcarrier Tablet (Microcarrier), is an independent innovation and the first pharmaceutical excipient grade microcarrier that can be used for cell drug development. It has obtained the certificate of analysis from relevant authoritative institutions such as National Institutes for Food and Drug Control, and obtained 2 qualifications for pharmaceutical excipients from the National Medical Products Administration (CDE approval registration number: F20210000003, F20200000496). Moreover, the product has obtained the DMF qualification for pharmaceutical excipients from U.S. FDA (DMF: 35481).
Products and services of CytoNiche can be widely used in the upstream process development of gene and cell therapy, extracellular vesicles, vaccines, and protein products. At the same time, it also has broad prospects for applications in the fields of regenerative medicine, organoids, and food technology (cell-cultured meat, etc.).
Our company has a R&D and transformation platform of 5,000 square meters, including a CDMO platform of more than 1,000 square meters, a GMP production platform of 4,000 square meters, and a new 1200 L microcarrier production line. The relevant technologies have obtained more than 100 patents and more than 30 articles about the technologies in international journals have been published. The core technology projects have obtained a number of national-level project support and applications.
Scan the QR code to read on your phone
-
Phone
- Service hotline+86 400-012-6688
-
E-mail
- E-mailwangal@cytoniche.com
- TOP



 京公网安备 11010802037749号
京公网安备 11010802037749号
